Neurovascular coupling over cortical brain areas and resting state network connectivity with and without rigidified carotid artery
- PMID: 39906907
- PMCID: PMC11792086
- DOI: 10.1117/1.NPh.12.S1.S14606
Neurovascular coupling over cortical brain areas and resting state network connectivity with and without rigidified carotid artery
Abstract
Significance: Neurovascular coupling (NVC) is key to research as hemodynamics can reflect neuronal activation and is often used in studies regarding the resting state network (RSN). However, several circumstances, including diseases that reduce blood vessel elasticity, can diminish NVC. In these cases, hemodynamic proxies might not accurately reflect the neuronal RSN.
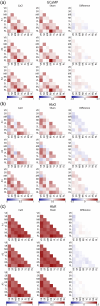
Aim: We aim to investigate in resting state if (1) NVC differs over brain regions, (2) NVC remains intact with a mild rigidification of the carotid artery, (3) hemodynamic-based RSN reflects neuronal-based RSN, and (4) RSN differs with a mildly rigidified artery.
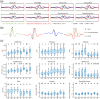
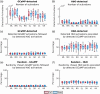
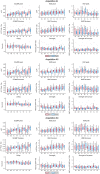
Approach: We rigidified the right common carotid artery of mice ( ) by applying a -soaked cloth to it (NaCl for Sham, ). With simultaneous GCaMP and intrinsic optical imaging, we compared neuronal activation to hemodynamic changes over the entire cortex.
Results: NVC parameters did not differ between the CaCl and Sham groups. Likewise, GCaMP and hemodynamic RSN showed similar connections in both groups. However, the parameters of NVC differed over brain regions. Retrosplenial regions had a slower response and a higher HbR peak than sensory and visual regions, and the motor cortex showed less HbO influx than sensory and visual regions.
Conclusions: NVC in a resting state differs over brain regions but is not altered by mild rigidification of the carotid artery.
Keywords: GCaMP; intrinsic optical imaging; neurovascular coupling; resting state.
© 2025 The Authors.
Figures









Similar articles
-
Alteration of functional connectivity despite preserved cerebral oxygenation during acute hypoxia.Sci Rep. 2023 Aug 15;13(1):13269. doi: 10.1038/s41598-023-40321-3. Sci Rep. 2023. PMID: 37582847 Free PMC article.
-
Altered cerebral neurovascular coupling in medication-overuse headache: A study combining multi-modal resting-state fMRI with 3D PCASL.Front Neurosci. 2023 Mar 15;17:1139086. doi: 10.3389/fnins.2023.1139086. eCollection 2023. Front Neurosci. 2023. PMID: 37008219 Free PMC article.
-
Association between carotid artery hemodynamics and neurovascular coupling in cerebral small vessel disease: an exploratory study.Front Aging Neurosci. 2025 Feb 7;17:1536552. doi: 10.3389/fnagi.2025.1536552. eCollection 2025. Front Aging Neurosci. 2025. PMID: 39990104 Free PMC article.
-
Neuronal networks and mediators of cortical neurovascular coupling responses in normal and altered brain states.Philos Trans R Soc Lond B Biol Sci. 2016 Oct 5;371(1705):20150350. doi: 10.1098/rstb.2015.0350. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27574304 Free PMC article. Review.
-
Nitric Oxide Pathways in Neurovascular Coupling Under Normal and Stress Conditions in the Brain: Strategies to Rescue Aberrant Coupling and Improve Cerebral Blood Flow.Front Physiol. 2021 Oct 22;12:729201. doi: 10.3389/fphys.2021.729201. eCollection 2021. Front Physiol. 2021. PMID: 34744769 Free PMC article. Review.
Cited by
-
Introduction to the Special Issue "Understanding Brain Disease with Live Imaging".Neurophotonics. 2025 Jan;12(Suppl 1):S14601. doi: 10.1117/1.NPh.12.S1.S14601. Epub 2025 Aug 29. Neurophotonics. 2025. PMID: 40894081 Free PMC article.
References
LinkOut - more resources
Full Text Sources

