Kinome Reprogramming of G2/M Kinases and Repression of MYCN Contribute to Superior Efficacy of Lorlatinib in ALK-Driven Neuroblastoma
- PMID: 39907037
- PMCID: PMC12322148
- DOI: 10.1158/1535-7163.MCT-24-0684
Kinome Reprogramming of G2/M Kinases and Repression of MYCN Contribute to Superior Efficacy of Lorlatinib in ALK-Driven Neuroblastoma
Abstract
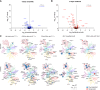
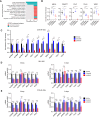
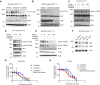
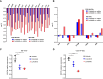
Mutations in the tyrosine kinase domain of the anaplastic lymphoma kinase (ALK) oncogene in neuroblastoma occur most frequently at one of three hotspot amino acid residues, with the F1174* and F1245* variants conferring de novo resistance to first- and second-generation ALK inhibitors, including crizotinib and ceritinib. Lorlatinib, a third-generation ALK/ROS1 inhibitor, overcomes de novo resistance and induces complete and sustained tumor regressions in patient-derived xenograft models unresponsive to crizotinib. Lorlatinib has now completed phase 1 testing in children and adults with relapsed/refractory ALK-driven neuroblastoma and entered pivotal phase 3 testing within the Children's Oncology Group. To define mechanisms underlying the superior activity of lorlatinib, we utilized a chemical proteomics approach to quantitatively measure functional kinome dynamics in response to lorlatinib and crizotinib in clinically relevant ALK-driven neuroblastoma patient-derived xenograft models. Lorlatinib was a markedly more potent inhibitor of ALK and preferentially downregulated several kinases implicated in G2/M cell-cycle transition compared with crizotinib. Lorlatinib treatment also led to the repression of MYCN expression and its occupancy at promoters of the same G2/M kinases. These data provide mechanistic insight into the superior efficacy of lorlatinib over crizotinib for the treatment of ALK-driven neuroblastoma.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
G.M. Witek reports grants from the NIH during the conduct of the study. E.R. Berko reports personal fees from Medison Pharma and Takeda Pharmaceuticals outside the submitted work. Y.P. Mossé reports grants from the NCI during the conduct of the study. No disclosures were reported by the other authors.
Figures


References
-
- Bhatia S, Tonorezos ES, Landier W. Survival of childhood cancer and subsequent clinical care-reply. JAMA 2024;331:617. - PubMed
-
- Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature 2008;455:971–4. - PubMed
-
- Janoueix-Lerosey I, Lequin D, Brugières L, Ribeiro A, de Pontual L, Combaret V, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 2008;455:967–70. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

