On the deprotonation of chlorothiazide
- PMID: 39907071
- PMCID: PMC11795655
- DOI: 10.1107/S2053229625000701
On the deprotonation of chlorothiazide
Abstract
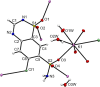
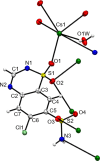
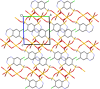
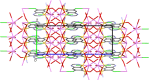
Three alkali metal salt forms of the diuretic chlorothiazide (systematic name: 6-chloro-1,1-dioxo-2H-1,2,4-benzothiazine-7-sulfonamide, HCTZ) are described. When crystallized from aqueous solution, the Na and K salts, namely, poly[[μ-aqua-aqua(μ3-6-chloro-1,1-dioxo-7-sulfamoyl-2H-1,2,4-benzothiadiazin-2-ido)sodium] hemihydrate], {[Na(C7H5ClN3O4S2)(H2O)2]·0.5H2O}n, and poly[[diaqua(μ5-6-chloro-1,1-dioxo-7-sulfamoyl-2H-1,2,4-benzothiadiazin-2-ido)potassium] hemihydrate], {[K(C7H5ClN3O4S2)(H2O)2]·0.5H2O}n, are both found to have stoichiometry MCTZ·2.5H2O, with CTZ deprotonated at a heterocyclic ring N atom. Both the stoichiometry and the deprotonation site are different to those described in previously published versions of these structures. The Cs salt form is found to be the monohydrate CsCTZ·H2O, namely, poly[[aqua(μ5-6-chloro-1,1-dioxo-7-sulfamoyl-2H-1,2,4-benzothiadiazin-2-ido)caesium], [Cs(C7H5ClN3O4S2)(H2O)]n. As with the Na and K cognates, this structure is also deprotonated at the heterocyclic ring. NaCTZ is found to be a two-dimensional coordination polymer with bridges between Na centres formed by H2O and SO2 groups, and by links through the length of the coordinated CTZ anions. Water ligands in KCTZ and CsCTZ are terminal, rather than bridging between metal centres, but both compounds form structures where M-Cl interactions link two-dimensional motifs formed via M-O bonds (and in CsCTZ, M-N bonds) into three-dimensional coordination polymers.
Keywords: alkali metals; crystal structure; diuretic; pharmaceuticals; salt selection; sulfonamide.
open access.
Figures


References
-
- Aljohani, M., Pallipurath, A. R., McArdle, P. & Erxleben, A. (2017). Cryst. Growth Des.17, 5223–5232.
-
- Bernal, I. & Watkins, S. F. (2013). Acta Cryst. C69, 808–810. - PubMed
-
- Cametti, M., Nissinen, M., Dalla Cort, A., Rissanen, K. & Mandolini, L. (2006). Inorg. Chem.45, 6099–6101. - PubMed
-
- Farrugia, L. J. (2012). J. Appl. Cryst.45, 849–854.
LinkOut - more resources
Full Text Sources
Research Materials

