Discovery of Hydrazine Clubbed Thiazoles as Potential Antidiabetic Agents: Synthesis, Biological Evaluation, and Molecular Docking Studies
- PMID: 39907170
- PMCID: PMC11795734
- DOI: 10.1002/ddr.70060
Discovery of Hydrazine Clubbed Thiazoles as Potential Antidiabetic Agents: Synthesis, Biological Evaluation, and Molecular Docking Studies
Abstract
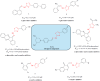
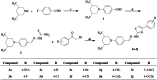
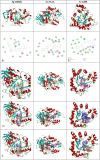
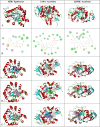
In this study, hydrazine clubbed thiazole derivatives (3a-3j) were obtained by Hantzsch thiazole synthesis and characterized by MS, 1H NMR, and 13C NMR. The inhibitory potentials of the derivatives against diabetes-related enzymes such as aldose reductase (AR), α-glycosidase (α-GLY), and α-amylase (α-AMY) were experimentally determined, and the results were supported by molecular docking. The results showed that the derivatives (3a-3j) displayed varied degree of potential inhibitory activity, with KI values covering the following ranges: 5.47 ± 0.53 to 23.89 ± 1.46 nM for AR and 1.76 ± 0.01 to 24.81 ± 0.15 μM for α-GLY, and with IC50 values 4.94-28.17 μM for α-AMY, as compared to standard epalrestat and acarbose (KI: 34.53 ± 2.52 nM for AR and 23.53 ± 2.72 μM for α-GLY, respectively). The selective activity of these derivatives on antidiabetic enzymes may be important for the treatment of diabetes and may lead to the development of alternative new compounds for this purpose.
Keywords: ADME; aldose reductase; alpha‐amylase; alpha‐glucosidase; antidiabetic; molecular docking; thiazole.
© 2025 Author(s). Drug Development Research published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Benzylidenehydrazine Derivatives: Synthesis, Antidiabetic Evaluation, Antioxidation, Mode of Inhibition, DFT and Molecular Docking Studies.Chem Biodivers. 2025 Mar;22(3):e202401556. doi: 10.1002/cbdv.202401556. Epub 2024 Nov 22. Chem Biodivers. 2025. PMID: 39530612 Free PMC article.
-
Unveiling anti-diabetic potential of new thiazole-sulfonamide derivatives: Design, synthesis, in vitro bio-evaluation targeting DPP-4, α-glucosidase, and α-amylase with in-silico ADMET and docking simulation.Bioorg Chem. 2024 Oct;151:107671. doi: 10.1016/j.bioorg.2024.107671. Epub 2024 Jul 23. Bioorg Chem. 2024. PMID: 39067419
-
Determination of the inhibition profiles of pyrazolyl-thiazole derivatives against aldose reductase and α-glycosidase and molecular docking studies.Arch Pharm (Weinheim). 2020 Dec;353(12):e2000118. doi: 10.1002/ardp.202000118. Epub 2020 Aug 6. Arch Pharm (Weinheim). 2020. PMID: 32761859
-
Discovery of novel thiazole derivatives containing pyrazole scaffold as PPAR-γ Agonists, α-Glucosidase, α-Amylase and COX-2 inhibitors; Design, synthesis and in silico study.Bioorg Chem. 2024 Nov;152:107760. doi: 10.1016/j.bioorg.2024.107760. Epub 2024 Aug 25. Bioorg Chem. 2024. PMID: 39197383
-
An insight on medicinal attributes of 1,2,3- and 1,2,4-triazole derivatives as alpha-amylase and alpha-glucosidase inhibitors.Mol Divers. 2024 Oct;28(5):3605-3634. doi: 10.1007/s11030-023-10728-1. Epub 2023 Sep 21. Mol Divers. 2024. PMID: 37733243 Review.
Cited by
-
Access to 2,5-Disubstituted Thiazoles Via Cyclization of N-Substituted α-Amino Acids.Org Lett. 2025 Jul 18;27(28):7513-7517. doi: 10.1021/acs.orglett.5c01807. Epub 2025 Jul 8. Org Lett. 2025. PMID: 40628372 Free PMC article.
References
-
- Akmal, M. , Patel P., and Wadhwa R.. 2024. “Alpha Glucosidase Inhibitors.” In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK557848/. - PubMed
-
- Ali, F. , Khan K. M., Salar U., et al. 2017. “Hydrazinyl Arylthiazole Based Pyridine Scaffolds: Synthesis, Structural Characterization, In Vitro α‐Glucosidase Inhibitory Activity, and In Silico Studies.” European Journal of Medicinal Chemistry 138: 255–272. 10.1016/j.ejmech.2017.06.041. - DOI - PubMed
-
- Ali, S. H. , and Sayed A. R.. 2021. “Review of the Synthesis and Biological Activity of Thiazoles.” Synthetic Communications 51, no. 5: 670–700. 10.1080/00397911.2020.1854787. - DOI
-
- Celestina, S. K. , Sundaram K., and Ravi S.. 2020. “In Vitro Studies of Potent Aldose Reductase Inhibitors: Synthesis, Characterization, Biological Evaluation and Docking Analysis of Rhodanine‐3‐hippuric Acid Derivatives.” Bioorganic Chemistry 97: 103640. 10.1016/j.bioorg.2020.103640. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

