The Stereoselectivity of Neighboring Group-Directed Glycosylation Is Concentration-Dependent
- PMID: 39907188
- PMCID: PMC11848824
- DOI: 10.1021/jacs.4c14402
The Stereoselectivity of Neighboring Group-Directed Glycosylation Is Concentration-Dependent
Abstract
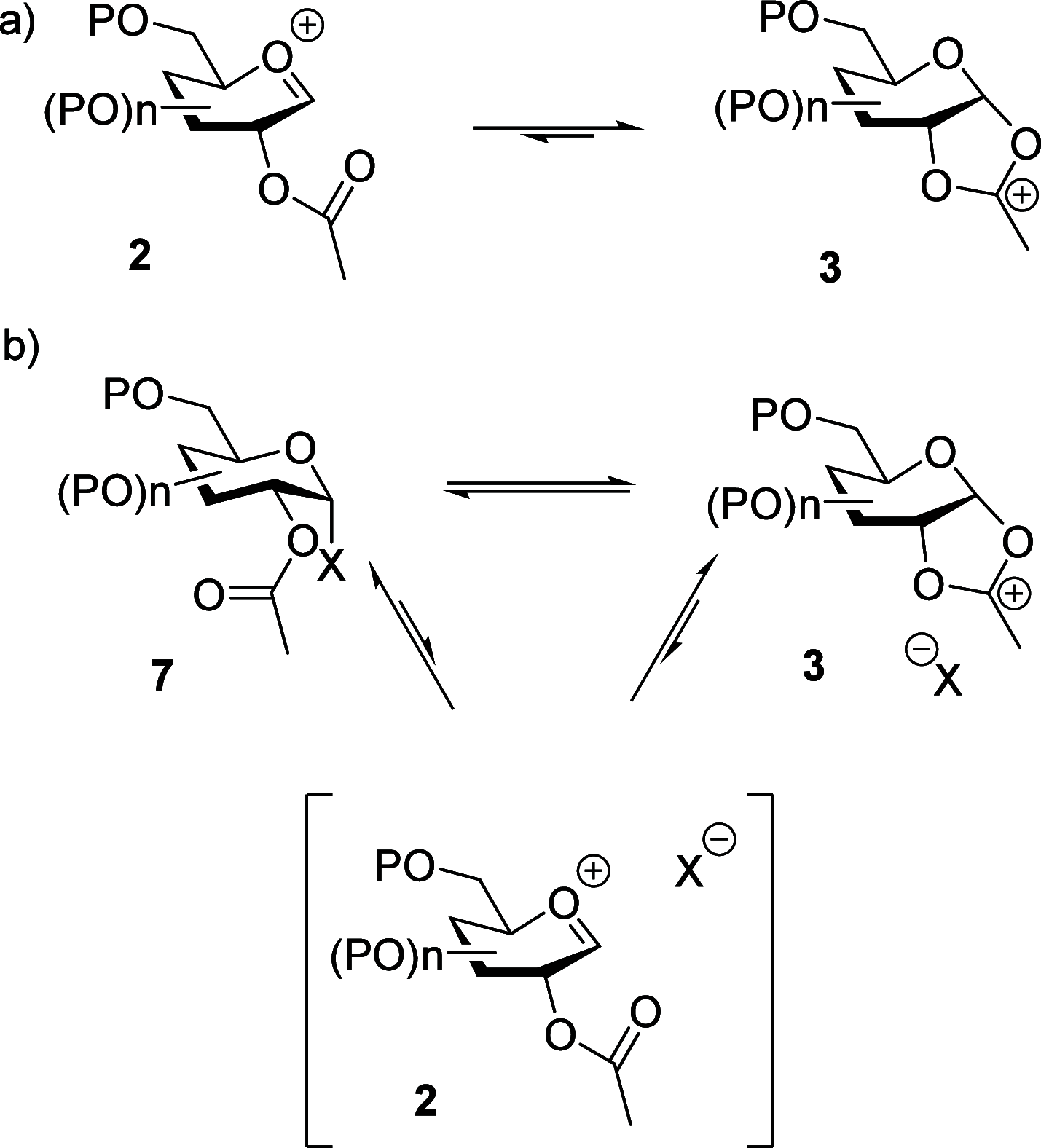
The formation of 1,2-trans-glycosides taking advantage of neighboring group participation by stereodirecting esters at the 2-position of glycosyl donors is widely held to be a robust and reliable protocol. Examples abound, however, of cases in which less-than-perfect selectivity is obtained, causing practitioners to survey different esters or resort to alternative strategies in the quest for optimal selectivities and yields. Consideration of the mechanism of neighboring group participation and in particular of the competing process of SN2-like glycosylation with activated covalent donors leads to the hypothesis that in cases of imperfect selectivity, more careful attention to reaction concentration and stoichiometry may be beneficial. Three case studies are presented to demonstrate the concentration dependence of neighboring group-directed glycosylation reactions targeting the formation of both 1,2-trans-equatorial and 1,2-trans-axial glycosides. Higher concentrations, whether achieved through increased acceptor:donor stoichiometry or through increased concentration at a fixed stoichiometry, mostly lead to erosion of 1,2-trans-selectivity as the competing SN2-like reaction with the covalent donors becomes increasingly important. These observations underline the importance of a rational, mechanism-based approach to glycosylation in general and more importantly suggest a simple approach to enhancing 1,2-trans-selectivity in neighboring group-directed glycosylation reactions displaying less-than-perfect 1,2-trans-selectivity, namely, moving to a different concentration regime.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Venturing beyond Donor-Controlled Glycosylation: New Perspectives toward Anomeric Selectivity.Acc Chem Res. 2018 Mar 20;51(3):628-639. doi: 10.1021/acs.accounts.7b00449. Epub 2018 Feb 22. Acc Chem Res. 2018. PMID: 29469568 Review.
-
Stereodirecting Effect of Esters at the 4-Position of Galacto- and Glucopyranosyl Donors: Effect of 4-C-Methylation on Side-Chain Conformation and Donor Reactivity, and Influence of Concentration and Stoichiometry on Distal Group Participation.J Org Chem. 2023 Oct 6;88(19):13883-13893. doi: 10.1021/acs.joc.3c01496. Epub 2023 Sep 7. J Org Chem. 2023. PMID: 37677151 Free PMC article.
-
DFT studies of the role of C-2-O-2 bond rotation in neighboring-group glycosylation reactions.Carbohydr Res. 2007 Jul 23;342(10):1291-304. doi: 10.1016/j.carres.2007.03.030. Epub 2007 Apr 14. Carbohydr Res. 2007. PMID: 17477909
-
A Stereoselective Glycosylation Approach to the Construction of 1,2-trans-β-d-Glycosidic Linkages and Convergent Synthesis of Saponins.Chemistry. 2022 Feb 1;28(7):e202104002. doi: 10.1002/chem.202104002. Epub 2021 Dec 22. Chemistry. 2022. PMID: 34859514
-
Recent advances in reagent-controlled stereoselective/stereospecific glycosylation.Carbohydr Res. 2019 Feb 1;473:72-81. doi: 10.1016/j.carres.2018.10.006. Epub 2018 Oct 22. Carbohydr Res. 2019. PMID: 30641292 Review.
Cited by
-
Stereoselective Approaches to β-Linked 2-Deoxy Sugars.Molecules. 2025 Apr 1;30(7):1578. doi: 10.3390/molecules30071578. Molecules. 2025. PMID: 40286171 Free PMC article. Review.
-
Elucidating the Curtin-Hammett Principle in Glycosylation Reactions: The Decisive Role of Equatorial Glycosyl Triflates.J Am Chem Soc. 2025 Jul 2;147(26):22597-22608. doi: 10.1021/jacs.5c03519. Epub 2025 Jun 23. J Am Chem Soc. 2025. PMID: 40548839 Free PMC article.
-
Efficient synthesis of O-glycosylated amino acids.RSC Chem Biol. 2025 May 7;6(6):851-856. doi: 10.1039/d5cb00076a. eCollection 2025 Jun 4. RSC Chem Biol. 2025. PMID: 40371313 Free PMC article.
References
-
- Koenigs W.; Knorr E. Derivatives of Dextrose. Sitzungsber. Bayr. Akad. Wiss. 1900, 103–105.
-
- Frush H. L.; Isbell H. S. Sugar Acetates, Acetylglycosyl Halides and Orthoacetates in Relation to the Walden Inversion. J. Research Natl. Bur. Standards 1941, 27, 413–428. 10.6028/jres.027.028. - DOI
-
- Winstein S.; Buckles R. E. The Role of Neighboring Groups in Replacement Reactions. 1. Retention of Configuration in the Reactions of Some Dihalides and Acetoxyhalides with Silver Acetate. J. Am. Chem. Soc. 1942, 64, 2780–2786. 10.1021/ja01264a020. - DOI
-
- Capon B. Mechanism in Carbohydrate Chemistry. Chem. Rev. 1969, 69, 407–498. 10.1021/cr60260a001. - DOI
-
- Capon B.; McManus S. P.. Neighboring Group Participation; Plenum, 1976.
Grants and funding
LinkOut - more resources
Full Text Sources

