Differential roles of Alzheimer's disease plasma biomarkers in stepwise biomarker-guided diagnostics
- PMID: 39907189
- PMCID: PMC11848384
- DOI: 10.1002/alz.14526
Differential roles of Alzheimer's disease plasma biomarkers in stepwise biomarker-guided diagnostics
Abstract
Introduction: This study aimed to investigate the differential roles of various plasma biomarkers in a stepwise diagnostic strategy for Alzheimer's disease (AD).
Methods: A total of 2984 participants, including 666 cognitively unimpaired (CU), 2032 with Alzheimer's clinical syndrome (ACS), and 286 non-ACS individuals, were recruited. Plasma amyloid beta (Aβ) 42/40, four phosphorylated tau (p-tau) epitopes, glial fibrillary acidic protein (GFAP), and neurofilament light chain (NfL) levels were measured using immunoassays.
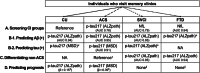
Results: NfL demonstrated fair to excellent accuracy in differentiating non-ACS from CU groups (area under the curve [AUC], 0.79 to 0.94). p-tau217 had the highest accuracy for identifying Aβ (AUC 0.94) and tau positron emission tomography status (AUC 0.91). In the ACS group, p-tau217 was the strongest predictor of cognitive decline (p < .001).
Discussion: NfL may serve as a useful screening tool, while p-tau217 is particularly valuable for confirming AD pathology and prognosis.
Highlights: Plasma NfL could screen for cognitive impairment. p-tau217 reliably detects AD pathology, regardless of diagnosis. p-tau217 and GFAP predict prognosis in ACS. Each plasma biomarker plays a distinct role in stepwise AD diagnostics.
Keywords: Alzheimer's disease; neurofilament light chain; plasma biomarker; positron emission tomography; p‐tau217.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
H.Z. has served on scientific advisory boards and/or as a consultant for AbbVie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has delivered lectures at symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant and on advisory boards for AbbVie, AC Immune, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd., Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served on data monitoring committees for Julius Clinical and Novartis; has delivered lectures, produced educational materials, and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai, and Roche Diagnostics; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. The other authors have no conflicts of interest to disclose. Author disclosures are available in the Supporting Information.
Figures



References
-
- Blennow K, Galasko D, Perneczky R, et al. The potential clinical value of plasma biomarkers in Alzheimer's disease. Alzheimers Dement. 2023;19:5805‐5816. - PubMed
-
- Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood‐based biomarkers for Alzheimer's disease: towards clinical implementation. Lancet Neurol. 2022;21:66‐77. - PubMed
MeSH terms
Substances
Grants and funding
- ZEN-21-848495/Alzheimer's Association 2021 Zenith Award
- AF-994551/Swedish Alzheimer Foundation
- 2019-02397/Swedish Research Council
- SG-23-1038904QC/Alzheimer's Association 2022-2025 Grant
- ADSF-21-831381-C/AD Strategic Fund and the Alzheimer's Association
- RS-2019-NR040057/National Research Foundation of Korea (NRF)
- 2017-00915/Swedish Research Council
- 2024-R001/Korean Dementia Association
- AF-939721/Swedish Alzheimer Foundation
- ALZ2022-0006/Bluefield Project, Cure Alzheimer's Fund, Olav Thon Foundation, Erling-Persson Family Foundation, Familjen Rönströms Stiftelse, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden
- ADSF-21-831376-C/AD Strategic Fund and the Alzheimer's Association
- FO2022-0270/Bluefield Project, Cure Alzheimer's Fund, Olav Thon Foundation, Erling-Persson Family Foundation, Familjen Rönströms Stiftelse, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden
- RS-2022-KH125667/Korea Health Industry Development Institute (KHIDI)
- 2024-ER1003-00/Korea National Institute of Health
- UKDRI-1003/National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL
- JPND2019-466-236/European Union Joint Programme - Neurodegenerative Disease Research
- ALFGBG-715986/Swedish State Support for Clinical Research
- P30 AG072980/AG/NIA NIH HHS/United States
- FO2017-0243/Bluefield Project, Cure Alzheimer's Fund, Olav Thon Foundation, Erling-Persson Family Foundation, Familjen Rönströms Stiftelse, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden
- 2023-00356/Swedish Research Council
- RS-2020-KH106434/Korea Dementia Research Center
- La Fondation Recherche Alzheimer (FRA), Paris, France, Kirsten and Freddy Johansen Foundation, Copenhagen, Denmark, and Familjen Rönströms Stiftelse, Stockholm, Sweden.
- 2022-01018/Swedish Research Council
- ADSF-24-1284328-C/AD Strategic Fund and the Alzheimer's Association
- 2022-00732/Swedish Research Council
- ALFGBG-71320/Swedish State Support for Clinical Research
- ADSF-21-831377-C/AD Strategic Fund and the Alzheimer's Association
- 101053962/European Union Horizon Europe research and innovation program
- AF-968270/Swedish Alzheimer Foundation
- JPND2021-00694/European Union Joint Programme - Neurodegenerative Disease Research
- 201809-2016862/Alzheimer Drug Discovery Foundation (ADDF)
- RS-2020-KH107436/Korea Dementia Research Center
- 860197/European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement
- #SMX1240561/Future Medicine 20*30 Project of the Samsung Medical Center
- AF-930351/Swedish Alzheimer Foundation
- RS-2021-II212068/Institute of Information & communications Technology Planning & Evaluation (IITP)
- ALFGBG-965240/Swedish State Support for Clinical Research
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

