Plasma Phosphorylated Tau 217 as a Discriminative Biomarker for Cerebral Amyloid Angiopathy
- PMID: 39907306
- PMCID: PMC11795418
- DOI: 10.1111/ene.70066
Plasma Phosphorylated Tau 217 as a Discriminative Biomarker for Cerebral Amyloid Angiopathy
Abstract
Background: Blood-based biomarkers may offer a non-invasive approach to diagnose cerebral amyloid angiopathy (CAA), especially in early-stage. We evaluated the ability of plasma phosphorylated tau-217 (p-tau 217) to differentiate CAA from Alzheimer's disease (AD) and deep perforator arteriopathy (DPA).
Methods: Patients with AD (age 73.7 ± 8.1 years), probable CAA (74.8 ± 6.9 years), or DPA (66.1 ± 10.4 years) were enrolled from memory and stroke clinics at a medical center in Taiwan. All participants received amyloid and tau PET scans. Plasma biomarkers were measured via a SIMOA immunoassay platform. The diagnostic utility of p-tau 217 was assessed using ROC analyses and the Youden cutoff. Associations between plasma p-tau 217 and neuroimaging variables in CAA were explored.
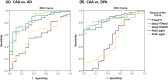
Results: Patients with CAA had lower plasma p-tau 217 (0.69 ± 0.76 vs. 1.28 ± 0.97 pg/mL, p < 0.001) and a lower p-tau 217/Aβ40 ratio (0.003 ± 0.002 vs. 0.006 ± 0.003, p < 0.001) than the AD group but higher levels than the DPA group (p-tau 217, 0.27 ± 0.13 pg/mL, p = 0.001; p-tau 217/Aβ40, 0.001 ± 0.0005, p < 0.001), although adjustment attenuated the difference in p-tau 217 between CAA and DPA. Plasma Aβ40, Aβ42, and Aβ40/Aβ42 were not significantly different between groups. Plasma p-tau 217 had moderate to good diagnostic utility to differentiate CAA vs. AD (sensitivity, 64.4%; specificity, 89.5%; AUC, 0.809) and CAA vs. DPA (sensitivity, 67.8%; specificity, 100%; AUC, 0.855). In CAA, p-tau 217 significantly correlated with the severity of CAA, amyloid PET signal intensity, and lobar microbleed count (p < 0.001).
Conclusions: Plasma p-tau 217 may represent a non-invasive biomarker for distinguishing cerebral amyloid angiopathy (CAA) from other conditions, including AD and DPA.
Keywords: Alzheimer disease; cerebral amyloid angiopathy; cerebral small vessel disease; phosphorylated tau 217; plasma biomarker.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Cerebral Amyloid Angiopathy and Downstream Alzheimer Disease Plasma Biomarkers.JAMA Netw Open. 2025 May 1;8(5):e258842. doi: 10.1001/jamanetworkopen.2025.8842. JAMA Netw Open. 2025. PMID: 40343697 Free PMC article.
-
CSF and plasma biomarkers in cerebral amyloid angiopathy: A single-center study and a systematic review/meta-analysis.Eur Stroke J. 2025 Mar;10(1):278-288. doi: 10.1177/23969873241260538. Epub 2024 Jun 13. Eur Stroke J. 2025. PMID: 38869035 Free PMC article.
-
Interest of CSF biomarker analysis in possible cerebral amyloid angiopathy cases defined by the modified Boston criteria.J Neurol. 2012 Nov;259(11):2429-33. doi: 10.1007/s00415-012-6520-8. Epub 2012 May 11. J Neurol. 2012. PMID: 22576334
-
Plasma biomarkers distinguish Boston Criteria 2.0 cerebral amyloid angiopathy from healthy controls.Alzheimers Dement. 2025 Mar;21(3):e70010. doi: 10.1002/alz.70010. Alzheimers Dement. 2025. PMID: 40156276 Free PMC article.
-
Core cerebrospinal fluid biomarker profile in cerebral amyloid angiopathy: A meta-analysis.Neurology. 2018 Feb 27;90(9):e754-e762. doi: 10.1212/WNL.0000000000005030. Epub 2018 Jan 31. Neurology. 2018. PMID: 29386280 Free PMC article. Review.
Cited by
-
Clinical Management of Cerebral Amyloid Angiopathy.J Clin Med. 2025 Jun 15;14(12):4259. doi: 10.3390/jcm14124259. J Clin Med. 2025. PMID: 40566003 Free PMC article. Review.
References
-
- Koemans E. A., Chhatwal J. P., van Veluw S. J., et al., “Progression of Cerebral Amyloid Angiopathy: A Pathophysiological Framework,” Lancet Neurology 22, no. 7 (2023): 632–642. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

