Anaemia predicts iron homoeostasis dysregulation and modulates the response to empagliflozin in heart failure with reduced ejection fraction: the EMPATROPISM-FE trial
- PMID: 39907687
- PMCID: PMC12011522
- DOI: 10.1093/eurheartj/ehae917
Anaemia predicts iron homoeostasis dysregulation and modulates the response to empagliflozin in heart failure with reduced ejection fraction: the EMPATROPISM-FE trial
Abstract
Background and aims: Sodium-glucose cotransporter 2 inhibitors (SGLT2i) impact iron metabolism in patients with heart failure but mechanisms are incompletely understood. This post hoc analysis explored interrelations between iron homeostasis, cardiac structure/function, exercise capacity, haematopoiesis, and sympathetic activity at baseline, and the effects of 6-month treatment with empagliflozin vs. placebo by anaemia status in EMPATROPISM-FE study participants.
Methods: Myocardial iron content (MIC, estimated by cardiac magnetic resonance T2* imaging), left ventricular (LV) volumes and LV ejection fraction (LVEF), exercise capacity, laboratory iron markers (LIM), haemoglobin/haematocrit, erythropoietin, and plasma norepinephrine were determined at baseline and 6 months.
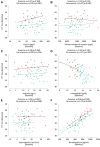
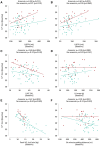
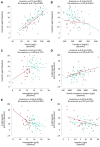
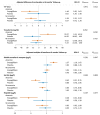
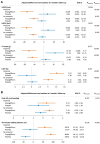
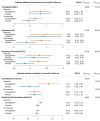
Results: At baseline, 24/80 participants (30%) had anaemia (haemoglobin < 13/<12 mg/dL in men/women). Patients with vs. without anaemia had higher T2* (indicating lower MIC, P < .001), lower peak oxygen consumption (VO2max, P = .024) and hepcidin (P = .017), and higher erythropoietin (P = .040) and norepinephrine (P = .016). Across subgroups, lower MIC correlated with higher LV volumes (P < .01) and norepinephrine (P < .001), and lower LVEF (P < .01), VO2max (P < .001) and haemoglobin/haematocrit (P < .001). Associations with LIM were poor (all P > .10). Empagliflozin increased MIC (P < .012), improved exercise capacity, and activated haematopoiesis. Changes in LIM and norepinephrine suggested progressive systemic iron depletion and sympatholysis. LV reverse remodelling was greater in individuals with anaemia.
Conclusions: Dysregulated cellular iron uptake/availability may be a shared mechanism in myocardial structural/functional impairment, reduced exercise capacity, and restricted haematopoiesis in heart failure, which are worse in patients with anaemia, and improve with empagliflozin. Empagliflozin increases MIC and decreases norepinephrine. Given this inverse association, sympatholysis may help explain the diverse cardiac and systemic benefits from SGLT2i therapy.
Clinical trial registration: NCT03485222 (www.clinicaltrials.gov).
Keywords: Anaemia; Cardiac remodelling; Empagliflozin; Exercise capacity; Heart failure; Iron homoeostasis; Neurohormonal activation.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

