Freeze-Dried Porous Collagen Scaffolds for the Repair of Volumetric Muscle Loss Injuries
- PMID: 39907689
- PMCID: PMC11897937
- DOI: 10.1021/acsbiomaterials.4c01601
Freeze-Dried Porous Collagen Scaffolds for the Repair of Volumetric Muscle Loss Injuries
Abstract
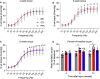
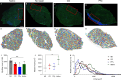
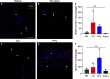
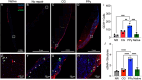
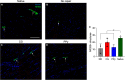
Volumetric muscle loss (VML) injuries are characterized by the traumatic loss of skeletal muscle, resulting in permanent damage to both tissue architecture and electrical excitability. To address this challenge, we previously developed a three-dimensional (3D) aligned collagen-glycosaminoglycan (CG) scaffold platform that supported in vitro myotube alignment and maturation. In this work, we assessed the ability of CG scaffolds to facilitate functional muscle recovery in a rat tibialis anterior (TA) model of VML. Functional muscle recovery was assessed following implantation of either nonconductive CG or electrically conductive CG-polypyrrole (PPy) scaffolds at 4, 8, and 12 weeks postinjury by in vivo electrical stimulation of the peroneal nerve. After 12 weeks, scaffold-treated muscles produced maximum isometric torque that was significantly greater than nontreated tissues. Histological analysis further supported these reparative outcomes with evidence of regenerating muscle fibers at the material-tissue interface in scaffold-treated tissues that were not observed in nonrepaired muscles. Scaffold-treated muscles possessed higher numbers of M1 and M2 macrophages at the injury, while conductive CG-PPy scaffold-treated muscles showed significantly higher levels of neovascularization as indicated by the presence of pericytes and endothelial cells, suggesting a persistent wound repair response not observed in nontreated tissues. Finally, only tissues treated with nonconductive CG scaffolds displayed neurofilament staining similar to native muscle, further corroborating isometric contraction data. Together, these findings show that both conductive and nonconductive CG scaffolds can facilitate improved skeletal muscle function and endogenous cellular repair, highlighting their potential use as therapeutics for VML injuries.
Keywords: CG-polypyrrole (PPy); collagen-glycosaminoglycan; volumetric muscle losss.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Update of
-
Freeze-dried porous collagen scaffolds for the repair of volumetric muscle loss injuries.bioRxiv [Preprint]. 2024 Sep 3:2024.08.30.610194. doi: 10.1101/2024.08.30.610194. bioRxiv. 2024. Update in: ACS Biomater Sci Eng. 2025 Mar 10;11(3):1598-1611. doi: 10.1021/acsbiomaterials.4c01601. PMID: 39282357 Free PMC article. Updated. Preprint.
References
-
- Baker H. B.; Passipieri J. A.; Siriwardane M.; Ellenburg M. D.; Vadhavkar M.; Bergman C. R.; Saul J. M.; Tomblyn S.; Burnett L.; Christ G. J. Cell and Growth Factor-Loaded Keratin Hydrogels for Treatment of Volumetric Muscle Loss in a Mouse Model. Tissue Eng., Part A 2017, 23, 572–584. 10.1089/ten.tea.2016.0457. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
