Development and evaluation of [11C]DPA-813 and [18F]DPA-814: novel TSPO PET tracers insensitive to human single nucleotide polymorphism rs6971
- PMID: 39907797
- PMCID: PMC12119672
- DOI: 10.1007/s00259-025-07109-1
Development and evaluation of [11C]DPA-813 and [18F]DPA-814: novel TSPO PET tracers insensitive to human single nucleotide polymorphism rs6971
Abstract
Purpose: The translocator protein 18 kDa (TSPO) is a widely used marker for imaging neuroinflammation via Positron Emission Tomography (PET). However, the vast majority of reported TSPO PET tracers display low binding affinity to a common isoform of human TSPO (rs6971; A147T), making them unsuitable for universal use in the general population. In this study, we have developed and preclinically validated two novel tracers designed to image TSPO in patients of all genotypes.
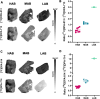
Methods: Novel analogues of known TSPO ligands were synthesised, evaluated for TSPO binding affinity in vitro (membranes prepared from transfected HEK-293T cells expressing wild-type (WT) or A147T TSPO) and radiolabelled with carbon-11 or fluorine-18. They were evaluated in situ (autoradiography on genotyped human brain tissue) and in vivo (rat, both WT and clinically relevant experimental autoimmune encephalomyelitis (EAE) neuroinflammation model) as potential polymorphism-insensitive TSPO PET tracers.
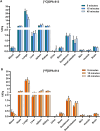
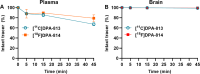
Results: Two new TSPO ligands, DPA-813 and DPA-814, displayed equivalent single-digit nanomolar binding affinities in vitro towards both human TSPO isoforms. [11C]DPA-813 and [18F]DPA-814 were synthesised in moderate radiochemical yields, high radiochemical purity, and high molar activity. Autoradiography on human MS tissues showed high specific binding for both tracers, irrespective of the TSPO isoform. The tracers demonstrated high plasma stability after 45 min and no brain metabolism with > 99% intact tracer. Biodistribution in WT animals indicated good brain uptake for both tracers (0.28 and 0.41%ID/g for [18F]DPA-814 and [11C]DPA-813, respectively). PET imaging in the clinically relevant EAE neuroinflammation model in rats showed significantly higher uptake of [11C]DPA-813 and [18F]DPA-814 in the spinal cord of the EAE rats compared to the controls.
Conclusion: We have developed two novel PET tracers that display indiscriminately high binding affinity to both common isoforms of human TSPO, show favourable metabolic stability and brain penetration in rats, and significantly higher uptake in the spinal cord of a neuroinflammatory rat model of multiple sclerosis. Going forward, first-in-human clinical validation will mark a critical juncture in the development of these tracers, which could offer substantial improvements over existing imaging tools for detecting neuroinflammation, irrespective of genetic variations.
Keywords: Autoradiography; Experimental autoimmune encephalomyelitis; Neuroinflammation; Polymorphism; Positron emission tomography; Translocator protein 18 kDa.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Animal experiments were performed under the ethical approval number AVD1140020185188, granted by the Central Committee for Animal Experimentation (CCD), The Netherlands. Competing interests: The authors have no relevant financial interests to disclose. ADW is editor-in-chief of Nuclear Medicine and Biology.
Figures
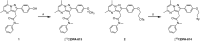

Similar articles
-
11C-DPA-713 Versus 18F-GE-180: A Preclinical Comparison of Translocator Protein 18 kDa PET Tracers to Visualize Acute and Chronic Neuroinflammation in a Mouse Model of Ischemic Stroke.J Nucl Med. 2019 Jan;60(1):122-128. doi: 10.2967/jnumed.118.209155. Epub 2018 Jul 5. J Nucl Med. 2019. PMID: 29976695 Free PMC article.
-
Radiosynthesis and characterization of [18F]BS224: a next-generation TSPO PET ligand insensitive to the rs6971 polymorphism.Eur J Nucl Med Mol Imaging. 2021 Dec;49(1):110-124. doi: 10.1007/s00259-021-05617-4. Epub 2021 Nov 16. Eur J Nucl Med Mol Imaging. 2021. PMID: 34783879 Free PMC article.
-
Synthesis and in vitro characterization of novel fluorinated derivatives of the translocator protein 18 kDa ligand CfO-DPA-714.Eur J Med Chem. 2017 Jan 5;125:346-359. doi: 10.1016/j.ejmech.2016.09.025. Epub 2016 Sep 9. Eur J Med Chem. 2017. PMID: 27688189
-
The potential of carbon-11 and fluorine-18 chemistry: illustration through the development of positron emission tomography radioligands targeting the translocator protein 18 kDa.J Labelled Comp Radiopharm. 2013 Mar-Apr;56(3-4):96-104. doi: 10.1002/jlcr.2992. J Labelled Comp Radiopharm. 2013. PMID: 24285315 Review.
-
Emerging TSPO-PET Radiotracers for Imaging Neuroinflammation: A Critical Analysis.Semin Nucl Med. 2024 Nov;54(6):856-874. doi: 10.1053/j.semnuclmed.2024.09.007. Epub 2024 Oct 29. Semin Nucl Med. 2024. PMID: 39477764 Review.
References
-
- Vivash L, O’Brien TJ. Imaging Microglial activation with TSPO PET: lighting up neurologic diseases? J Nucl Med. 2016;57:165–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

