Blood Sample Collection in Randomized Controlled Trials for Biomarker Discovery and Validation: Experience of the PREOPANC-2 Trial
- PMID: 39907876
- PMCID: PMC12130148
- DOI: 10.1245/s10434-025-16890-0
Blood Sample Collection in Randomized Controlled Trials for Biomarker Discovery and Validation: Experience of the PREOPANC-2 Trial
Abstract
Background: This study aimed to investigate the feasibility and yield of blood sample collection in an investigator-initiated nationwide randomized controlled trial (RCT).
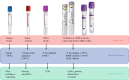
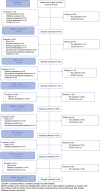
Methods: In the PREOPANC-2 trial, 375 patients with (borderline) resectable pancreatic cancer were randomly assigned to two neoadjuvant regiments in 19 centers in the Netherlands (2018-2021). Blood sample collection was scheduled at seven time points before, during, and after treatment. The primary outcome was the proportion of successfully collected blood samples at each scheduled time point.
Results: Of the 375 randomized patients, 12 were excluded from blood sample collection before any treatment. From the remaining 363 patients, 1513 (87 %) of 1748 blood samples were collected, processed, mailed, and centrally stored. The blood samples were collected before treatment from 347 (96 %) of the 363 patients, after the first neoadjuvant cycle from 322 (94 %) of 343 patients, after neoadjuvant treatment (i.e., before surgery) from 260 (83 %) of 313 patients, and after surgery from 210 (77 %) of 271 patients. During the follow-up visits, blood samples were collected from 147 (82 %) of 179 patients 12 months after randomization and from 83 (77 %) of 108 patients after 24 months. A total of 220 samples (13 %) were missing. The most common causes for missing blood samples were scheduling oversights, unsuccessful blood draw attempts, and mailing failures (151 times, 69 %). Blood sample collection was canceled 69 times (31 %) due to COVID-19.
Conclusion: Blood sample collection in the PREOPANC-2 trial had a yield of 96 % before treatment and an overall yield of 87 %. Collection of blood samples for biomarker studies is feasible in a nationwide RCT.
Keywords: Biomarkers; Pancreatic cancer; Personalized medicine; Randomized controlled trial.
© 2025. The Author(s).
Conflict of interest statement
Disclosure: Eileen M. O’Reilly received research funding for the institution from Genentech/Roche, BioNTech, AstraZeneca, Arcus, Elicio, Parker Institute, NIH/NCI, and Digestive Care. She is consulting/DSMB for Arcus, Ability Pharma, Alligator Bioscience, BioNTech, Ipsen, Merck, Novartis, Syros, Leap Therapeutics, Astellas, Autem, BMS, Revolution Medicine, Tempus, Fibrogen, Merus, Revi Agios (spouse), Genentech-Roche (spouse), Eisai (spouse), and Servier (Spouse). Johanna W. Wilmink is consulting/advisor for MSD, Servier, Novartis, Astra Zeneca, and Nordic. She has received research funding for the institution from Servier, Nordic, and MSD. Casper H. J. van Eijck is consultant for AIM ImunnoTech. The remaining authors have no conflicts of interest.
Figures
References
-
- Ashley EA. Towards precision medicine. Nat Rev Genet. 2016;17:507–22. - PubMed
-
- Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2019;20:71–88. - PubMed
-
- La Thangue NB, Kerr DJ. Predictive biomarkers: a paradigm shift towards personalized cancer medicine. Nat Rev Clin Oncol. 2011;8:587–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical