The mere-measurement effect of patient-reported outcomes: a systematic review and meta-analysis
- PMID: 39907942
- PMCID: PMC12064450
- DOI: 10.1007/s11136-025-03909-y
The mere-measurement effect of patient-reported outcomes: a systematic review and meta-analysis
Abstract
Purpose: The mere-measurement effect is the phenomenon in which subjects exposed to measurements have their perceptions and/or behaviors on the inquired topic affected simply through the act of responding. Patient-reported outcomes (PROs) are increasingly used to assess patient perspective and quality of life in clinical trials and different health care settings. This systematic literature review aims to assess what is currently known about the mere-measurement effect of PROs.
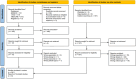
Methods: A systematic literature review and meta-analysis was conducted. We included studies that provided evidence on perceptual or behavioral changes in patients as a result of exposure to questionnaire items assessing PROs. All adult participants were included regardless of demographics. Any study design was considered eligible for inclusion. The databases MEDLINE [PubMed], CINAHL [Ebsco], Web of Science and ScienceDirect were searched.
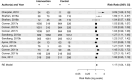
Results: The search resulted in 636 articles which led to a final extraction of nine. Overall, seven of the nine articles reported a significant main effect, i.e. presence of the mere-measurement effect. For the meta-analysis, thirteen different interventions were included. There was a one-directional, positive and significant overall risk ratio of 1.17 [CI95% 1.04;1.30].
Conclusion: This systematic review found significant potential for the mere-measurement effect to shape respondents' behaviors or perceptions for the better, opening the door to the possibility of engineering PROs to serve as a subtle intervention. Future considerations and directions for research are discussed.
Keywords: Mere-measurement effect; Patient-reported outcomes; Question-behaviour effect; Systematic literature review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: TS reports personal fees from AOP Health, AbbVie, Pfizer, Roche, and Takeda, outside the submitted work. Ethics approval: Due to the nature of this study no ethics approval was required.
Figures
References
-
- Meregaglia, M., Malandrini, F., Angelini, S., & Ciani, O. (2023). The assessment of patient-reported outcomes for the authorisation of medicines in Europe: A Review of European Public Assessment Reports from 2017 to 2022. Applied Health Economics and Health Policy,21(6), 925–935. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous