Clinical Pharmacokinetics of Oral ALZ-801/Valiltramiprosate in a 2-Year Phase 2 Trial of APOE4 Carriers with Early Alzheimer's Disease
- PMID: 39907966
- PMCID: PMC11954699
- DOI: 10.1007/s40262-025-01482-8
Clinical Pharmacokinetics of Oral ALZ-801/Valiltramiprosate in a 2-Year Phase 2 Trial of APOE4 Carriers with Early Alzheimer's Disease
Abstract
Introduction: ALZ-801/valiltramiprosate is an oral, small-molecule inhibitor of β-amyloid (Aβ) oligomer formation in late-stage development as a potential disease-modifying therapy for Alzheimer's disease (AD). ALZ-801, a valine-conjugated prodrug, is rapidly converted to tramiprosate after oral dosing. Upon conversion to tramiprosate, it generates a single metabolite, 3-sulfopropanoic acid (3-SPA). Both tramiprosate and 3-SPA are active anti-Aβ oligomer agents that mediate ALZ-801's central mechanism of action (MOA). We summarize herein the pharmacokinetics (PK) of ALZ-801 in apolipoprotein ε4 (APOE4) carrier subjects with early AD from a phase 2 trial.
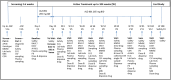
Methods: The ALZ-801 phase 2 study was designed to evaluate longitudinal effects of ALZ-801 (265 mg BID) on plasma, cerebrospinal fluid (CSF) and volumetric magnetic resonance imaging (MRI) AD biomarkers, and clinical outcomes over 104 weeks in APOE4 carriers with early AD. Eighty-four subjects (31 APOE4/4 homozygotes and 53 APOE3/4 heterozygotes) with positive CSF biomarkers of amyloid and tau pathology were enrolled. The phase 2 study included a substudy of 24 subjects to provide 8-h steady-state PK at 65 weeks. Sparse PK samples were also analyzed. The relationships between plasma PK exposure and clinical characteristics [i.e., sex, APOE genotype, age, body mass index (BMI), estimated glomerular filtration rate (eGFR), concomitant acetylcholinesterase inhibitor (AChEI) use, and tablet lot] were evaluated.
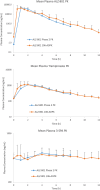
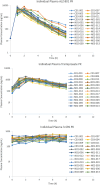
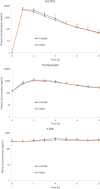
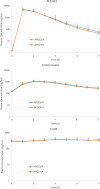
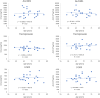
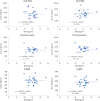
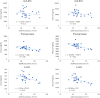
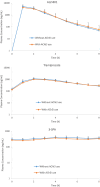
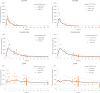
Results: The steady-state plasma PK results were closely aligned with the previous 2-week PK in the ALZ-801 phase 1b study in APOE4 carrier subjects with AD, as well as a phase 1 7-day PK study in heathy elderly volunteers. Following oral dosing, ALZ-801 was rapidly converted to the active moieties, tramiprosate and 3-SPA. The intersubject variability in plasma drug levels was low, confirming the superior performance of ALZ-801 versus oral tramiprosate tablet (150 mg BID) from the earlier tramiprosate phase 3 trials. Correlation analysis versus clinical characteristics showed that plasma exposures (Cmax and AUC8h) for ALZ-801, tramiprosate, and 3-SPA were not affected by sex, APOE genotype, age, BMI, concomitant AChEI use, or tablet lot. Plasma exposures of both tramiprosate and 3-SPA, but not ALZ-801, were inversely correlated with eGFR, in line with renal excretion as the primary route of elimination. ALZ-801 was well tolerated without new safety signals or events of amyloid-related imaging abnormalities (ARIA).
Conclusions: The steady-state PK profile of oral ALZ-801 in subjects with early AD was not affected by sex, APOE genotype, age, BMI, concomitant use of AChEI, or tablet lot. The inverse relationship of plasma exposures of tramiprosate and 3-SPA, but not ALZ-801, versus eGFR is consistent with renal clearance as the primary route of elimination for tramiprosate and 3-SPA (active moieties), and with the efficient conversion of ALZ-801 prodrug to the active moieties after dosing. These results demonstrate that ALZ-801 displays favorable PK properties without evidence of interactions with demographic characteristics and support its development as an oral disease-modifying treatment for AD.
Trial registration: https://clinicaltrials.gov/study/NCT04693520 .
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: The clinical study and analyses in this report were supported by Alzheon, Inc. Conflict of interest: John A. Hey, Jeremy Y. Yu, Susan Abushakra, Jean F. Schaefer, Aidan Power, Patrick Kesslak, Jijo Paul and Martin Tolar are employees of Alzheon, Inc. All authors own Alzheon stocks and/or stock options. Ethics approval: The protocol was approved by the assigned Ethics Committees (EC) in the Netherlands and in Czech Republic and by the institutional EC in the Czech Republic. These include the Medical-Ethical Review Committee (METC), 12 October 2020; Ethics Committee of the Fakultní nemocnice Brno, 14 October 2020; Ethics Committee of the Fakultní nemocnice v Motole, 09 September 2020; Ethics Committee of the Fakultní nemocnice u sv Anny v Brne, 09 September 2020; and Etická Komise Vestra Clinics, 17 September 2020. Consent to participate: All subjects and their partners, caregivers or legal representatives signed informed consent before participation in the study. Consent for publication: All subjects and their partners, caregivers or legal representatives provided consent that the data are publishable before participation in the study. Availability of data and material: The data and material contained in this publication are proprietary to Alzheon Inc. Code availability: Not applicable. Author contributions: JAH wrote the article in collaboration with JYY and JFS; SA, JAH, AP, PK, JP and MT were involved in the design and conduct of the ALZ-801-201 ADBM study. All authors have read and approved the final submitted manuscript and agree to be accountable for the work.
Figures
References
-
- Qaseem A, Snow V, Cross JT Jr, Forciea MA, Hopkins R Jr, Shekelle P, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008;148(5):370–8. 10.7326/0003-4819-148-5-200803040-00008. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

