Microbial Contamination of Percutaneous Endoscopic Gastrostomy Tube and Nasogastric Tube with Drug-Resistant Bacteria
- PMID: 39907997
- PMCID: PMC12136516
- DOI: 10.1159/000543972
Microbial Contamination of Percutaneous Endoscopic Gastrostomy Tube and Nasogastric Tube with Drug-Resistant Bacteria
Abstract
Introduction: Enteral nutrition is used in patients with stroke, head-and-neck or esophageal cancer surgery, or repeated aspiration pneumonia. Japanese enteral nutrition guidelines recommend percutaneous endoscopic gastrostomy tube (PEG) for long-term use of >4 weeks and nasogastric tube (NGT) for short-term use of <4 weeks. Catheters may be contaminated with microorganisms because enteral feeding products passed through them daily, but there are few reports on catheter contamination in Japan and no reports on the duration of catheter use. There are also reports that enteral feeding is a risk factor for the appearance of drug-resistant bacteria. Therefore, this study aimed to determine whether microorganisms could be isolated from enteral feeding catheters and determine the percentage of drug-resistant bacteria.
Methods: Forty-six PEGs and 59 NGTs were collected at Showa University Hospital and Showa University Fujigaoka Rehabilitation Hospital from May 2019 to March 2020. Microorganisms were cultured by incubating 20 mL pass/wash solution of sterile purified water on BHI agar medium (37°C) for 24-72 h. The strains were isolated and cultured, then frozen (80°C) and stored. Antimicrobial susceptibility was determined by broth microdilution method.
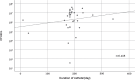
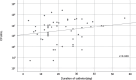
Results: Microorganisms were detected in 37 PEGs and 57 NGTs (p = 0.007). Bacteria were detected in 27 PEGs and 53 NGTs (p < 0.001), and yeasts were detected in 29 PEGs and 28 NGTs (p = 0.112). Drug-resistant bacteria were isolated from 19.6% (9 of 46) in PEGs and 23.7% (14 of 59) in NGTs.
Conclusions: PEGs and NGTs were contaminated with microorganisms, and drug-resistant bacteria were isolated. This study provides a rationale for future appropriate use in enteral feeding catheters.
Keywords: Bacteria; Drug-resistant bacteria; Enteral feeding; Nasogastric tube; Percutaneous endoscopic gastrostomy.
© 2025 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- JASPEN , Guidelines for venous enteral nutrition. 3rd ed. Tokyo: Shorinsha; 2016. Available from: https://minds.jcqhc.or.jp/common/wp-content/plugins/pdfjs-viewer-shortco...
-
- Okuma T, Nakamura M, Totake H, Fukunaga Y. Microbial contamination of enteral feeding formulas and diarrhea. Nutrition. 2000;16(9):719–22. - PubMed
-
- Sano M, Shindo Y, Takahashi K, Okumura J, Sakakibara T, Murakami Y, et al. Risk factors for antibiotic resistance in hospital-acquired and ventilator-associated pneumonia. J Infect Chemother. 2022;28(6):745–52. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical