Simvastatin and Rifaximin in Decompensated Cirrhosis: A Randomized Clinical Trial
- PMID: 39908052
- PMCID: PMC11800124
- DOI: 10.1001/jama.2024.27441
Simvastatin and Rifaximin in Decompensated Cirrhosis: A Randomized Clinical Trial
Abstract
Importance: There are no useful treatments to prevent the development of severe complications of liver cirrhosis. Simvastatin and rifaximin have shown beneficial effects in liver cirrhosis.
Objective: To assess whether simvastatin combined with rifaximin improves outcomes in patients with decompensated cirrhosis.
Design, setting, and participants: Double-blind, placebo-controlled, phase 3 trial conducted among patients with decompensated cirrhosis in 14 European hospitals between January 2019 and December 2022. The last date of follow-up was December 2022.
Interventions: Patients were randomly assigned to receive simvastatin, 20 mg/d, plus rifaximin, 1200 mg/d (n = 117), or identical-appearing placebo (n = 120) for 12 months in addition to standard therapy, stratified according to Child-Pugh class B or C.
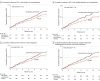
Main outcomes and measures: The primary end point was incidence of severe complications of liver cirrhosis associated with organ failure meeting criteria for acute-on-chronic liver failure. Secondary outcomes included transplant or death and a composite end point of complications of cirrhosis (ascites, hepatic encephalopathy, variceal bleeding, acute kidney injury, and infection).
Results: Among the 237 participants randomized (Child-Pugh class B: n = 194; Child-Pugh class C: n = 43), 72% were male and the mean age was 57 years. There were no differences between the 2 groups in terms of development of acute-on-chronic liver failure (21 [17.9%] vs 17 [14.2%] patients in the treatment and placebo groups, respectively; hazard ratio, 1.23; 95% CI, 0.65-2.34; P = .52); transplant or death (22 [18.8%] vs 29 [24.2%] patients in the treatment and placebo groups, respectively; hazard ratio, 0.75; 95% CI, 0.43-1.32; P = .32); or development of complications of cirrhosis (50 [42.7%] vs 55 [45.8%] patients in the treatment and placebo groups, respectively; hazard ratio, 0.93; 95% CI, 0.63-1.36; P = .70). Incidence of adverse events was similar in both groups (426 vs 419; P = .59), but 3 patients in the treatment group (2.6%) developed rhabdomyolysis.
Conclusions and relevance: The addition of simvastatin plus rifaximin to standard therapy does not improve outcomes in patients with decompensated liver cirrhosis.
Trial registration: ClinicalTrials.gov Identifier: NCT03780673.
Conflict of interest statement
Figures

References
-
- Sepanlou SG, Safiri S, Bisignano C, et al. ; GBD 2017 Cirrhosis Collaborators . The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245-266. doi: 10.1016/S2468-1253(19)30349-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

