Remote Monitoring of Chemotherapy-Induced Peripheral Neuropathy by the NeuroDetect iOS App: Observational Cohort Study of Patients With Cancer
- PMID: 39908091
- PMCID: PMC11840369
- DOI: 10.2196/65615
Remote Monitoring of Chemotherapy-Induced Peripheral Neuropathy by the NeuroDetect iOS App: Observational Cohort Study of Patients With Cancer
Abstract
Background: Chemotherapy-induced peripheral neuropathy (CIPN) is a common and debilitating adverse effect of neurotoxic chemotherapy characterized by symptoms such as numbness, tingling, and weakness. Effective monitoring and detection of CIPN are crucial for avoiding progression to irreversible symptoms. Due to the inconvenience of clinic-based objective assessment, CIPN detection relies primarily on patients' reporting of subjective symptoms, and patient-reported outcomes are used to facilitate CIPN detection. Our previous study found evidence that objective functional assessments completed within a smartphone app may differentiate patients with and those without CIPN after treatment.
Objective: This prospective, longitudinal observational cohort study aimed to determine the feasibility and accuracy of app-based remote monitoring of CIPN in patients with cancer undergoing neurotoxic chemotherapeutic treatment and to conduct exploratory comparisons of app-based functional CIPN monitoring versus patient-reported outcome-only monitoring.
Methods: The NeuroDetect app (Medable Inc) includes subjective EORTC (European Organization for Research and Treatment of Cancer) Quality of Life Questionnaire (QLQ)-20-item scale (CIPN20) and 6 objective functional assessments that use smartphone sensors to mimic neurological examinations, such as walking, standing, and manual dexterity tests. The functional assessment data were collected from patients with cancer undergoing neurotoxic chemotherapy, and a neurological examination was conducted at the end of treatment to diagnose CIPN in the feet (CIPN-f) or CIPN in the hands (CIPN-h). Various classification models including NeuroDetect features only (NeuroDetect Model) CIPN20-only (CIPN20 Model) or a combination of both (Combined Model) were trained and evaluated for accuracy in predicting CIPN probability.
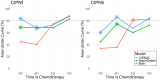
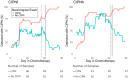
Results: Of the 45 patients who completed functional assessments and neurological examinations, 24 had CIPN-f, and 29 had CIPN-h. The NeuroDetect Model could discriminate between patients with and those without CIPN-f (area under the curve=83.8%, comparison with no information rate P=.02) but not CIPN-h (area under the curve=67.9%, P=.18). The rolling rotation features from the eyes-closed phase of the Romberg Stance assessment showed the greatest contribution to CIPN-f (40% of total variable importance) and the Finger Tapping assessment showed the greatest contribution to CIPN-h (85% of total variable importance). The NeuroDetect Model had numerically, and at some time points statistically, superior performance to the CIPN20 Model in both CIPN-f and CIPN-h, particularly before and early in treatment. The Combined Model numerically, though not statistically, outperformed either assessment strategy individually, indicating that the combination of functional and patient-reported assessment within a smartphone may be optimal to CIPN detection.
Conclusions: Our findings demonstrate the feasibility of integrating subjective and objective CIPN assessment into a smartphone app for remote, longitudinal CIPN monitoring. Studies of larger patient cohorts are needed to refine the app-based CIPN detection models and determine whether their use in practice improves CIPN detection.
Keywords: CIPN; application; balance; chemotherapy; chemotherapy-induced peripheral neuropathy; detection; gait; mHealth; manual dexterity; mobile health; mobile phone; monitoring; neurotoxic chemotherapy; patient-reported outcomes; peripheral neuropathy; smartphone.
©Ciao-Sin Chen, Michael P Dorsch, Sarah Alsomairy, Jennifer J Griggs, Reshma Jagsi, Michael Sabel, Amro Stino, Brian Callaghan, Daniel L Hertz. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 05.02.2025.
Conflict of interest statement
Conflicts of Interest: BC receives research and editorial support from the American Academy of Neurology, consults for DynaMed, and performs medical legal work including the vaccine injury compensation program. Unrelated to the current work, RJ has stock options as compensation for her advisory board role in Equity Quotient, a company that evaluates culture in health care companies; she has received personal fees from the Greenwall Foundation, Doris Duke Charitable Foundation, the National Institutes of Health, the Blue Cross Blue Shield Association, Physicians Education Resource, and the American Medical Association; and grants or contracts from the National Institutes of Health, the Doris Duke Charitable Foundation, the American Cancer Society, and the Susan G. Komen Foundation. She has served as an expert witness for Kleinbard, LLC and Hawks Quindel Law.
Figures


References
-
- Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi: 10.1016/j.pain.2014.09.020. https://linkinghub.elsevier.com/retrieve/pii/00006396-201412000-00006 00006396-201412000-00006 - DOI - PubMed
-
- Winters-Stone KM, Horak F, Jacobs PG, Trubowitz P, Dieckmann NF, Stoyles S, Faithfull S. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2017;35(23):2604–2612. doi: 10.1200/JCO.2016.71.3552. https://europepmc.org/abstract/MED/28586243 - DOI - PMC - PubMed
-
- Bandos H, Melnikow J, Rivera DR, Swain SM, Sturtz K, Fehrenbacher L, Wade JL, Brufsky AM, Julian TB, Margolese RG, McCarron EC, Ganz PA. Long-term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG oncology/NSABP B-30. J Natl Cancer Inst. 2018;110(2):djx162. doi: 10.1093/jnci/djx162. https://europepmc.org/abstract/MED/28954297 4093779 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

