CO2 potentiates echinocandin efficacy during invasive candidiasis therapy via dephosphorylation of Hsp90 by Ptc2 in condensates
- PMID: 39908105
- PMCID: PMC11831212
- DOI: 10.1073/pnas.2417721122
CO2 potentiates echinocandin efficacy during invasive candidiasis therapy via dephosphorylation of Hsp90 by Ptc2 in condensates
Abstract
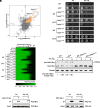
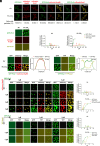
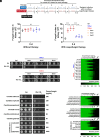
Carbon dioxide is a signaling cue critical for fungal pathogenesis. Ptc2, a type 2C protein phosphatase (PP2C), serves as a conserved CO2 sensor in fungi. By combining phosphoproteomic and biochemical assays, we identified Hsp90 as a direct target of Ptc2 at host CO2 concentrations and Ssb1 as a Ptc2 target protein regardless of CO2 levels in Candida albicans, the most prevalent human fungal pathogen. Ptc2 forms reversible condensates at elevated CO2, which enables the recruitment of Hsp90, but not Ssb1, to condensates, allowing efficient dephosphorylation. This process confers an enhanced susceptibility to caspofungin in vitro and during in vivo infection therapy. Importantly, we demonstrate this phenomenon in non-albicans Candida species. Sequential passages of C. albicans in mice with caspofungin treatment readily induce in vivo drug tolerance, causing therapeutic failure. These evolved strains display increased resistance to caspofungin under host concentrations of CO2 but remain susceptible in air. Collectively, our study reveals a profound impact of host concentrations of CO2 on antifungal drug susceptibility and connects this phenotype to therapeutic outcomes and highlights condensate formation as an efficient means that enables selective recruitment of substrates for certain signaling events.
Keywords: Candida; Hsp90; carbon dioxide; drug susceptibility; phase separation.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



Similar articles
-
Core histones govern echinocandin susceptibility in Candida glabrata.Microbiol Spectr. 2025 Jun 3;13(6):e0239924. doi: 10.1128/spectrum.02399-24. Epub 2025 Apr 30. Microbiol Spectr. 2025. PMID: 40304478 Free PMC article.
-
Regulation of phosphatidylinositol-(4,5)-bisphosphate and active-Rho1p levels and distribution is crucial for correct spatio-temporal cytokinesis and echinocandin responses in Candida albicans.Antimicrob Agents Chemother. 2025 Jun 4;69(6):e0190024. doi: 10.1128/aac.01900-24. Epub 2025 May 5. Antimicrob Agents Chemother. 2025. PMID: 40323423 Free PMC article.
-
Non-antifungal medications administered during fungal infections drive drug tolerance and resistance in Candida albicans.J Med Microbiol. 2025 Jul;74(7). doi: 10.1099/jmm.0.002046. J Med Microbiol. 2025. PMID: 40720257
-
Treatment and prophylaxis of invasive candidiasis with anidulafungin, caspofungin and micafungin and its impact on use and costs: review of the literature.Eur J Med Res. 2011 Apr 28;16(4):180-6. doi: 10.1186/2047-783x-16-4-180. Eur J Med Res. 2011. PMID: 21486732 Free PMC article.
-
Activity of rezafungin against Candida auris.J Antimicrob Chemother. 2025 Jun 3;80(6):1482-1493. doi: 10.1093/jac/dkaf124. J Antimicrob Chemother. 2025. PMID: 40304092 Free PMC article. Review.
Cited by
-
Tolerance and heteroresistance to echinocandins in Candida auris: conceptual issues, clinical implications, and outstanding questions.mSphere. 2025 May 27;10(5):e0016125. doi: 10.1128/msphere.00161-25. Epub 2025 Apr 16. mSphere. 2025. PMID: 40237528 Free PMC article. Review.
-
Bacterial metabolites induce cell wall remodeling, antifungal resistance, and immune recognition of commensal fungi.bioRxiv [Preprint]. 2025 Jul 27:2025.07.26.666966. doi: 10.1101/2025.07.26.666966. bioRxiv. 2025. PMID: 40777320 Free PMC article. Preprint.
References
-
- Denning D. W., Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 24, e428–e438 (2024). - PubMed
-
- Salmanton-Garcia J., et al. , Attributable mortality of candidemia–Results from the ECMM Candida III multinational European Observational Cohort Study. J. Infect. 89, 106229 (2024). - PubMed
-
- Pappas P. G., et al. , Clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 62, 409–417 (2016). - PubMed
-
- Pfaller M. A., Moet G. J., Messer S. A., Jones R. N., Castanheira M., Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: Report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J. Clin. Microbiol. 49, 396–399 (2011). - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 32170089/MOST | National Natural Science Foundation of China (NSFC)
- 82373493/MOST | National Natural Science Foundation of China (NSFC)
- 32070074/MOST | National Natural Science Foundation of China (NSFC)
- 82173429/MOST | National Natural Science Foundation of China (NSFC)
- 32470075/MOST | National Natural Science Foundation of China (NSFC)
LinkOut - more resources
Full Text Sources

