Neuronal α-synuclein toxicity is the key driver of neurodegeneration in multiple system atrophy
- PMID: 39908177
- PMCID: PMC12233558
- DOI: 10.1093/brain/awaf030
Neuronal α-synuclein toxicity is the key driver of neurodegeneration in multiple system atrophy
Abstract
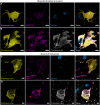
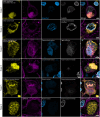
Multiple system atrophy (MSA) is a rare, rapidly progressing neurodegenerative disorder often misdiagnosed as Parkinson's disease (PD). Although both conditions share some clinical features, MSA is distinct in its pathological hallmark: oligodendroglial cytoplasmic α-synuclein (α-Syn) inclusions, known as glial cytoplasmic inclusions. These glial cytoplasmic inclusions are pathognomonic for MSA, but they do not lead to significant oligodendroglial cell loss. Instead, MSA is characterized by a substantially greater loss of non-dopaminergic neurons in the nigrostriatal and olivopontocerebellar systems compared with PD. This widespread neuronal degeneration, which is not seen to the same extent in PD, plays a crucial role in the clinical presentation of MSA and is important to consider if PD is to be redefined as a neuronal α-Syn disease. It also raises the question of differences in the potential toxicity of lesions in MSA and the underlying cause of neuronal death in MSA. By combining an N-terminus α-Syn antibody that reveals more α-Syn pathology and super-resolution microscopy, we identified α-Syn fibrils in MSA neurons penetrating the nucleus from the cytoplasm, leading to nuclear destruction and neuronal death. Our data indicate an early invasion of neuronal nuclei by α-Syn pathology in MSA, precipitating rapid nuclear envelope destruction, as observed through significant structural damage, including the loss of Lamin integrity. Although the progression of α-Syn pathology from the cytoplasm to the nucleus might be similar in oligodendroglia and neurons, the aggregation state of the α-Syn proteoforms involved differs because proteolytic resistance of α-Syn inclusions is significantly higher in neurons, and the nucleus is destroyed. We describe the progressive impact of α-Syn nuclear pathology on MSA neurons and show that this is a more detrimental and rapid pathology driving neurodegeneration. Our data suggest that oligodendroglial inclusions contain more soluble, less toxic α-Syn proteoforms, consistent with two distinct α-Syn filaments in MSA. We propose renaming MSA as a neuronal nuclear and oligodendroglial α-synucleinopathy to reflect these two distinct pathologies better.
Keywords: multiple system atrophy; neuronal inclusions; neuronal synucleinopathy; nuclear inclusions; oligodendroglial inclusions; α-synuclein.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors have no competing interests to disclose.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

