Transient ocular hypertension remodels astrocytes through S100B
- PMID: 39908332
- PMCID: PMC11798533
- DOI: 10.1371/journal.pone.0313556
Transient ocular hypertension remodels astrocytes through S100B
Abstract
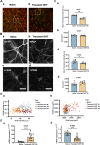
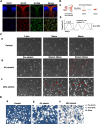
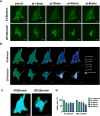
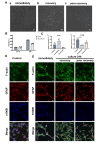
Glaucoma is a series of irreversible and progressive optic nerve degenerations, often accompanied by astrocyte remodeling as the disease progresses, a process that is insufficiently understood. Here, we investigated the morphology of retinal and optic nerve head (ONH) astrocytes under mechanical stress, and explored whether a specific phase is present that precedes astrocyte remodeling. A mouse model of transient ocular hypertension (OHT) and an in vitro cell stretch model were established to mimic the pathological conditions of increased intraocular pressure and mechanical stress on cultured cells. Glial fibrillary acidic protein (GFAP), S100B, and actin staining were used to characterize astrocyte morphology and cytoskeleton, with qPCR used to measure mRNA expression. We also silenced S100B expression and conduct RNA sequencing on ONH astrocytes. Astrocytes displayed weaker GFAP intensity (p < 0.0001) in the early-stage OHT mouse model, prior to the onset of hypertrophy, which was accompanied by an increase in GFAP mRNA expression (p < 0.0001) and a decrease in S100B mRNA expression (p < 0.001). In vitro-stretched astrocytes tended to contract and had fewer cellular processes and more elongated cell bodies. Downregulation of S100B expression occurred in in both the in vivo (p = 0.0001) and in vitro (p = 0.0023) models. S100B-silenced ONH astrocytes were similarly characterized by a slender morphology. In the RNA-seq analysis, genes downregulated by more than fivefold were predominantly enriched in terms related to nutrient metabolism, motor proteins and morphogenesis. Meanwhile, genes upregulated by more than fivefold were primarily associated with terms related to histone modification and visual perception. As an early response to mechanical stress, S100B expression is downregulated in astrocytes, which assume a slender morphology, reminiscent of cell "weakening." Silencing intracellular S100B expression induced similar morphology changes and altered the transcriptome. Stress-induced changes were reversible, with evidence of enhanced late-stage reactivation that is likely related to S100B.
Copyright: © 2025 Huang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma.J Neuroinflammation. 2012 May 14;9:92. doi: 10.1186/1742-2094-9-92. J Neuroinflammation. 2012. PMID: 22583833 Free PMC article.
-
Optic Nerve Head Astrocytes Display Axon-Dependent and -Independent Reactivity in Response to Acutely Elevated Intraocular Pressure.Invest Ophthalmol Vis Sci. 2019 Jan 2;60(1):312-321. doi: 10.1167/iovs.18-25447. Invest Ophthalmol Vis Sci. 2019. PMID: 30665231 Free PMC article.
-
Piezo1 plays a role in optic nerve head astrocyte reactivity.Exp Eye Res. 2021 Mar;204:108445. doi: 10.1016/j.exer.2021.108445. Epub 2021 Jan 16. Exp Eye Res. 2021. PMID: 33465396 Free PMC article.
-
Elevated pressure induced astrocyte damage in the optic nerve.Brain Res. 2008 Dec 9;1244:142-54. doi: 10.1016/j.brainres.2008.09.044. Epub 2008 Sep 24. Brain Res. 2008. PMID: 18848926
-
Bioinformatic and statistical analysis of the optic nerve head in a primate model of ocular hypertension.BMC Neurosci. 2008 Sep 26;9:93. doi: 10.1186/1471-2202-9-93. BMC Neurosci. 2008. PMID: 18822132 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

