A detailed theory of thalamic and cortical microcircuits for predictive visual inference
- PMID: 39908384
- PMCID: PMC11800772
- DOI: 10.1126/sciadv.adr6698
A detailed theory of thalamic and cortical microcircuits for predictive visual inference
Abstract
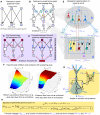
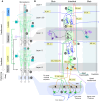
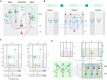
Understanding cortical microcircuitry requires theoretical models that can tease apart their computational logic from biological details. Although Bayesian inference serves as an abstract framework of cortical computation, precisely mapping concrete instantiations of computational models to biology under real-world tasks is necessary to produce falsifiable neural models. On the basis of a recent generative model, recursive cortical networks, that demonstrated excellent performance on vision benchmarks, we derive a theoretical cortical microcircuit by placing the requirements of the computational model within biological constraints. The derived model suggests precise algorithmic roles for the columnar and laminar feed-forward, feedback, and lateral connections, the thalamic pathway, blobs and interblobs, and the innate lineage-specific interlaminar connectivity within cortical columns. The model also explains several visual phenomena, including the subjective contour effect and neon-color spreading effect, with circuit-level precision. Our model and methodology provides a path forward in understanding cortical and thalamic computations.
Figures







Similar articles
-
Canonical microcircuits for predictive coding.Neuron. 2012 Nov 21;76(4):695-711. doi: 10.1016/j.neuron.2012.10.038. Neuron. 2012. PMID: 23177956 Free PMC article. Review.
-
Looking back: corticothalamic feedback and early visual processing.Trends Neurosci. 2006 Jun;29(6):298-306. doi: 10.1016/j.tins.2006.05.002. Epub 2006 May 19. Trends Neurosci. 2006. PMID: 16712965 Review.
-
Dendritic Excitability and Gain Control in Recurrent Cortical Microcircuits.Cereb Cortex. 2015 Oct;25(10):3561-71. doi: 10.1093/cercor/bhu200. Epub 2014 Sep 9. Cereb Cortex. 2015. PMID: 25205662 Free PMC article.
-
Differences in visually induced MEG oscillations reflect differences in deep cortical layer activity.Commun Biol. 2020 Nov 25;3(1):707. doi: 10.1038/s42003-020-01438-7. Commun Biol. 2020. PMID: 33239652 Free PMC article.
-
The role of thalamic population synchrony in the emergence of cortical feature selectivity.PLoS Comput Biol. 2014 Jan;10(1):e1003418. doi: 10.1371/journal.pcbi.1003418. Epub 2014 Jan 9. PLoS Comput Biol. 2014. PMID: 24415930 Free PMC article.
Cited by
-
Integrated world modeling theory expanded: Implications for the future of consciousness.Front Comput Neurosci. 2022 Nov 24;16:642397. doi: 10.3389/fncom.2022.642397. eCollection 2022. Front Comput Neurosci. 2022. PMID: 36507308 Free PMC article.
References
-
- D. Marr, Vision: A Computational Investigation into the Human Representation and Processing of Visual Information (MIT Press, 1982).
-
- Markram H., Muller E., Ramaswamy S., Reimann M. W., Abdellah M., Sanchez C. A., Ailamaki A., Alonso-Nanclares L., Antille N., Arsever S., Kahou G. A. A., Berger T. K., Bilgili A., Buncic N., Chalimourda A., Chindemi G., Courcol J. D., Delalondre F., Delattre V., Druckmann S., Dumusc R., Dynes J., Eilemann S., Gal E., Gevaert M. E., Ghobril J. P., Gidon A., Graham J. W., Gupta A., Haenel V., Hay E., Heinis T., Hernando J. B., Hines M., Kanari L., Keller D., Kenyon J., Khazen G., Kim Y., King J. G., Kisvarday Z., Kumbhar P., Lasserre S., le Bé J. V., Magalhães B. R. C., Merchán-Pérez A., Meystre J., Morrice B. R., Muller J., Muñoz-Céspedes A., Muralidhar S., Muthurasa K., Nachbaur D., Newton T. H., Nolte M., Ovcharenko A., Palacios J., Pastor L., Perin R., Ranjan R., Riachi I., Rodríguez J. R., Riquelme J. L., Rössert C., Sfyrakis K., Shi Y., Shillcock J. C., Silberberg G., Silva R., Tauheed F., Telefont M., Toledo-Rodriguez M., Tränkler T., van Geit W., Díaz J. V., Walker R., Wang Y., Zaninetta S. M., De Felipe J., Hill S. L., Segev I., Schürmann F., Reconstruction and simulation of neocortical microcircuitry. Cell 163, 456–492 (2015). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

