Phase 2 trial of ibrutinib and nivolumab in patients with relapsed CNS lymphomas
- PMID: 39908461
- PMCID: PMC11985036
- DOI: 10.1182/bloodadvances.2024014635
Phase 2 trial of ibrutinib and nivolumab in patients with relapsed CNS lymphomas
Abstract
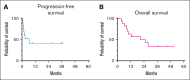
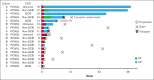
Treatment options are limited for both relapsed/refractory primary and secondary central nervous system (CNS) lymphoma and the prognosis remains poor. Previous studies have shown the activity of Bruton tyrosine kinase inhibitors and programmed death-1-targeted therapies in CNS lymphoma, and studies suggested potential synergy. Therefore, we conducted a phase 2 trial that combined ibrutinib with nivolumab for patients with relapsed/refractory CNS lymphoma. Patients received 560 mg oral ibrutinib daily with 240 mg IV nivolumab every 14 days (28 days per cycle). Patients who had partial or complete response after 6 cycles of treatment could continue therapy for up to 2 years unless progression or unacceptable toxicity occurred. A total of 18 patients were enrolled with a median age of 63 years (range, 43-88). The median number of previous lines of therapy was 2 (range, 1-4); 55% had refractory disease, 17% previously underwent stem cell transplant, and 11% previously underwent chimeric antigen receptor T-cell therapy. The best overall response rate was 78% and the best complete response rate was 50% (95% confidence interval, 26-74). The median progression-free survival and overall survival was 6.5 months and 21.0 months, respectively, and 3 patients continued to be in remission for >2 years. Treatment was generally well tolerated but 2 patients stopped treatment because of fatigue. Ibrutinib and nivolumab had reasonable safety and clinical activity in patients with refractory/relapsed CNS lymphoma and warrants further investigation. This trial was registered at www.ClinicalTrials.gov as #NCT03770416.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: D.C. reports receiving research funding from Genentech, Bristol Myers Squibb (BMS), Genmab, MorphoSys, and ONO Pharmaceutical. R.E.S. reports receiving research funding from BMS, GlaxoSmithKline (GSK), and Rafael Pharmaceuticals. R.N. reports serving as advisory board member/consultant for 280 Bio, Inc. S.A. reports receiving research funding from Nektar, Merck, Xencor, Chimagen and Genmab, Kite/Gilead, Janssen, and Caribou; serving as a member of the scientific advisory committee for Chimagen; serving on the data safety monitoring board for Myeloid Therapeutics; and serving as an advisory board member or consultant for ADC Therapeutics and Kite/Gilead. P.S. reports receiving research funding from Sobi, AstraZeneca-Acerta, ALX Oncology, and ADC Therapeutics, and serving as an advisory board member/consultant for Roche-Genentech, AbbVie-Genmab, Ipsen, Kite/Gilead, Hutchison MediPharma, AstraZeneca-Acerta, ADC Therapeutics, Sobi, and TG Therapeutics. L.M. reports receiving research funding from Dizal Pharma and BMS. S.P.I. reports receiving research funding from Merck, Innate, AstraZeneca, Dren Bio, CRISPR Therapeutics, Legend, Pfizer, ONO Pharmaceutical, Acrotech, and Corvus; serving as an advisory board member or consultant for Salarius, Electra, Seagen, Dren Bio, Electra, and Secura Bio; and owning stock in IMPaRT.ai. L.J.N. reports receiving research funding from BMS, Caribou Biosciences, Daiichi Sankyo, Genentech, Genmab, Gilead/Kite, Janssen, Incyte, Ipsen, Merck, Novartis, and Takeda, and serving as an advisory board member or consultant for AbbVie, BMS, Caribou Biosciences, Genentech, Genmab, Gilead/Kite, Janssen, Incyte, Ipsen, Merck, Novartis, Regeneron, and Takeda. S.S.N. reports receiving research support from Kite/Gilead, BMS, Allogene, Precision Biosciences, Adicet Bio, Sana Biotechnology, and Cargo Therapeutics; serving as an advisory board member or consultant for Kite/Gilead, Merck, Sellas Life Sciences, Athenex, Allogene, Incyte, Adicet Bio, BMS, bluebird bio, Fosun Kite, Sana Biotechnology, Caribou, Astellas Pharma, MorphoSys, Janssen, Chimagen, ImmunoACT, Orna Therapeutics, Takeda, Synthekine, CARsgen, Appia Bio, GSK, Galapagos, ModeX Therapeutics, and Jazz Pharmaceuticals; having stock options from Longbow Immunotherapy, Inc; and owing intellectual property related to cell therapy. J.R.W. reports receiving research funding from ADC Therapeutics, Allogene, AstraZeneca, BMS, Genentech, Janssen, Kite/Gilead, MorphoSys/Incyte, Novartis, and Nurix; and serving as an advisory board member or consultant for AbbVie, ADC Therapeutics, Allogene, AstraZeneca, BMS, Genentech, Genmab, Janssen, Kite/Gilead, MorphoSys/Incyte, Novartis, Nurix, Pfizer, Regeneron. The remaining authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical