Intermediate-dose posttransplantation cyclophosphamide for myeloablative HLA-haploidentical bone marrow transplantation
- PMID: 39908565
- PMCID: PMC12148395
- DOI: 10.1182/bloodadvances.2024014879
Intermediate-dose posttransplantation cyclophosphamide for myeloablative HLA-haploidentical bone marrow transplantation
Abstract
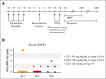
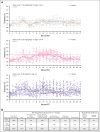
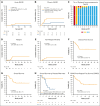
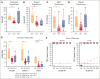
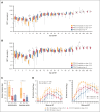
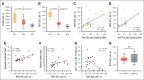
High-dose posttransplantation cyclophosphamide (HD-PTCy), given at 50 mg/kg/day on days +3/+4, is a standard-of-care graft-versus-host disease (GVHD) prophylaxis for allogeneic hematopoietic cell transplantation (HCT). Our murine MHC-haploidentical HCT studies suggested intermediate-dose PTCy produces superior GVHD control compared with HD-PTCy and PTCy is maximally effective on day +4. We conducted a single-institutional prospective phase 1/2 trial to reduce PTCy dosing to 25 mg/kg/day on days +3/+4 or on day +4 only for myeloablative HLA-haploidentical bone marrow HCT using PTCy, sirolimus, and mycophenolate mofetil. Among 35 patients, 89% were ethnic/racial minorities, 46% had high/very-high-risk disease, and median comorbidity score was 3. The phase 1 dose-limiting-toxicity, grade III-IV acute GVHD, was not observed after either reduced-PTCy dose level. PTCy 25 mg/kg/day on days +3/+4 (intermediate-dose (ID)-PTCy; n = 23), the phase 2 dose, resulted in no grade II-IV acute GVHD; 2-year cumulative incidences of chronic GVHD requiring systemic immunosuppression, nonrelapse mortality, and relapse were 13%, 17%, and 22%, and 2-year overall survival, disease-free survival, and GVHD-free/relapse-free survival were 61%, 61%, and 52%. In exploratory analysis compared with HD-PTCy (n = 5), ID-PTCy resulted in significantly faster engraftment and T-cell reconstitution, fewer transfusions, less mucositis, and reduced severity of BK-virus-associated cystitis/urethritis; area-under-the-curve exposure of 4-hydroxycyclophosphamide (4HCY), a key cyclophosphamide metabolite, correlated with these outcomes but not with chronic GVHD occurrence. Ideal-body-weight-based PTCy dosing best approximated 4HCY exposure. ID-PTCy is effective and has apparent clinical benefits compared with HD-PTCy. Before broader implementation, further studies are needed to confirm these findings and define optimal PTCy dosing across various donor/graft types. This trial was registered at www.clinicaltrials.gov as #NCT03983850.
Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
References
-
- O'Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8(7):377–386. - PubMed
-
- Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15(5):1767–1777. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

