Differential antibody response to EBV proteome following EBVST immunotherapy in EBV-associated lymphomas
- PMID: 39908567
- PMCID: PMC11995064
- DOI: 10.1182/bloodadvances.2024014937
Differential antibody response to EBV proteome following EBVST immunotherapy in EBV-associated lymphomas
Abstract
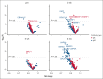
Epstein-Barr virus (EBV) is associated with a diverse range of lymphomas. EBV-specific T-cell (EBVST) infusions have shown promise in safety and clinical effectiveness in treating EBV-associated lymphomas; however, not all patients respond to T-cell immunotherapies. To identify EBV antigen-specific antibody responses associated with clinical outcomes, we comprehensively characterized antibody responses to the complete EBV proteome using a custom protein microarray in 56 patients with EBV-associated lymphoma who received EBVST infusions in phase 1 clinical trials. Responders (nonprogressors) and nonresponders (progressors) had distinct antibody profiles against EBV. Twenty-five immunoglobulin G (IgG) antibodies were significantly elevated in higher levels in nonresponders than in responders at 3 months after EBVST infusion. Ten of these remained significant after adjustment for sex, age, and cancer type, including LMP2A (4 variants), BGRF1/BDRF1 (2 variants), LMP1, BKRF2, BKRF4, and BALF5. Random forest analysis identified these 10 IgG antibodies as key predictors of clinical response. Paired analyses using blood samples collected at both before infusion and 3 months after EBVST infusion indicated an increase in the mean antibody level for 6 other anti-EBV antibodies (IgG [BGLF2, LF1, and BGLF3]; IgA [BGLF3, BALF2, and BBLF2/3) in nonresponders. Overall, our findings suggest that these EBV-directed antibodies as potential serological markers for predicting clinical responses to EBVST infusions and as therapeutic targets for immunotherapy in EBV-positive lymphomas. These trials were registered at www.clinicaltrials.gov as #NCT01555892 (Cytotoxic T-Lymphocytes for EBV-positive Lymphoma [GRALE]), #NCT02973113 (Nivolumab With Epstein Barr Virus Specific T Cells [EBVSTS], Relapsed/Refractory EBV Positive Lymphoma [PREVALE]), and #NCT02287311 (Most Closely Matched 3rd Party Rapidly Generated LMP, BARF1, and EBNA1 Specific CTL, EBV-Positive Lymphoma [MABEL]).
Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution.
Conflict of interest statement
Conflict-of-interest disclosure: H.E.H. has equity in AlloVir and Marker Therapeutics, and has served on the advisory boards for Tessa Therapeutics, March Biosciences, and Fresh Wind Biotechnologies. C.M.R. has equity in AlloVir and Marker Therapeutics; has served on the advisory boards for Tessa Therapeutics and Marker Therapeutics; has received research support from Tessa Therapeutics; and her spouse has interests in Walking Fish Therapeutics, Abintus, Allogene, Memgen, Turnstone Biologics, Coya Therapeutics, TScan Therapeutics, Oncimmune, and Poseida Therapeutics. Z.L. is currently employed by Merck & Co, Inc. The remaining authors declare no competing financial interests.
Figures



References
-
- Hudnall SD, Ge Y, Wei L, Yang N-P, Wang H-Q, Chen T. Distribution and phenotype of Epstein–Barr virus-infected cells in human pharyngeal tonsils. Mod Pathol. 2005;18(4):519–527. - PubMed
-
- Middeldorp JM, Brink AATP, van den Brule AJC, Meijer CJLM. Pathogenic roles for Epstein–Barr virus (EBV) gene products in EBV-associated proliferative disorders. Crit Rev Oncol Hematol. 2003;45(1):1–36. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

