Glucagon-like peptide 1 receptor agonists and venous thromboembolism in type 2 diabetes: a target trial emulation
- PMID: 39908569
- PMCID: PMC12142527
- DOI: 10.1182/bloodadvances.2025015871
Glucagon-like peptide 1 receptor agonists and venous thromboembolism in type 2 diabetes: a target trial emulation
Abstract
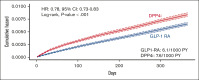
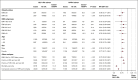
Glucagon-like peptide 1 receptor agonists (GLP1-RA) are antidiabetic agents recently approved for weight loss. Obesity is an established risk factor for venous thromboembolism (VTE). Moreover, preclinical studies have shown that GLP1-RA may attenuate thromboxane-induced platelet activation. Therefore, we hypothesized that GLP1-RA use may reduce the risk of VTE. We performed a target trial emulation (TTE) using a population-based database of electronic health records to evaluate whether GLP1-RA use is associated with a reduction in VTE in patients with type 2 diabetes mellitus (T2DM) compared with dipeptidyl peptidase-4 inhibitors (DPP4i). Patients who were newly initiated on GLP1-RA were propensity score matched to patients who were newly initiated on DPP4i. We evaluated the primary outcome, composite VTE, identified using ICD-10 (International Classification of Diseases, Tenth Revision) codes, within 12 months of the initiation of GLP1-RA or DPP4i. The study cohort comprised 540 258 patients with 270 129 individuals receiving either GLP1-RA or DPP4i. Over 12 months of follow-up, patients who received GLP1-RA had a lower incidence of VTE compared with patients who received DPP4i (6.1 vs 7.6 events per 1000 patient-years; hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.73-0.83). This was similar for pulmonary embolism (2.9 vs 3.8 events per 1000 patient-years; HR, 0.74; 95% CI, 0.68-0.82) and deep vein thrombosis (3.9 vs 4.7 events per 1000 patient-years; HR, 0.81; 95% CI, 0.75-0.88). In this propensity score-matched, TTE study, patients with T2DM who received a GLP1-RA had a lower risk of VTE at 1 year compared with patients who received DPP4i.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: K.A.B. reports consulting fees from Sanofi, outside the submitted work. R.P. reports consulting fees from Merck Research, outside the submitted work. The remaining authors declare no competing financial interests.
Figures



References
-
- ISTH Steering Committee for World Thrombosis Day Thrombosis: a major contributor to the global disease burden. J Thromb Haemostasis. 2014;12(10):1580–1590. - PubMed
-
- Kahn SR, Comerota AJ, Cushman M, et al. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;130(18):1636–1661. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

