Hsa-miR-532-3p protects human decidual mesenchymal stem cells from oxidative stress in recurrent spontaneous abortion via targeting KEAP1
- PMID: 39908863
- PMCID: PMC11847473
- DOI: 10.1016/j.redox.2025.103508
Hsa-miR-532-3p protects human decidual mesenchymal stem cells from oxidative stress in recurrent spontaneous abortion via targeting KEAP1
Abstract
Background: Human decidual mesenchymal stem cells (hDMSCs) play crucial roles in pregnancy. The decreased resistance of hDMSCs to oxidative stress is a key factor contributing to recurrent spontaneous abortion (RSA). miRNAs have essential functions in the proliferation and apoptosis of decidual tissues. However, the miRNAs involved in regulating oxidative stress in hDMSCs remain unclear.
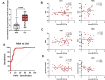
Methods: Decidual tissues and hDMSCs were collected from patients with RSA and early pregnancy miscarriages. We assessed the antioxidant capacity of hDMSCs in both groups by detecting relevant indicators. Furthermore, differentially expressed miRNAs in hDMSCs were analyzed through miRNA sequencing. We evaluated the interaction between hsa-miR-532-3p and KEAP1 using a luciferase reporter assay. A mouse model of RSA was constructed for confirmation. Finally, we analyzed the correlations between serum hsa-miR-532-3p levels and the clinical features of pregnant women with RSA.
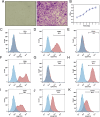
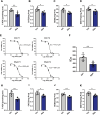
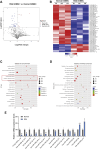
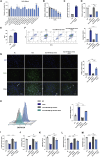
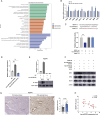
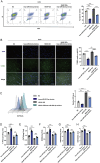
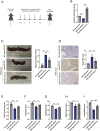
Results: miRNA sequencing revealed 44 miRNAs whose expression was downregulated and 9 miRNAs whose expression was upregulated in hDMSCs from the RSA group compared with those from the control group. The overexpression of hsa-miR-532-3p led to a significantly increased antioxidant capacity in hDMSCs. The knockdown or overexpression of hsa-miR-532-3p led to the upregulation or downregulation of KEAP1 expression, respectively. In a mouse model, the overexpression of hsa-miR-532-3p reduced embryo absorption rates in RSA mice, decreased KEAP1 expression levels in decidual tissues, and concurrently enhanced the resistance to oxidative stress. Furthermore, in patients diagnosed with RSA, serum hsa-miR-532-3p levels were significantly and negatively correlated with the gestational age.
Conclusions: Our study revealed a lower expression level of hsa-miR-532-3p in the hDMSCs of patients with RSA. Moreover, hsa-miR-532-3p protects hDMSCs from oxidative stress by targeting the Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 (KEAP1/NRF2) pathway. Hsa-miR-532-3p is closely related to gestational age and has good predictive value for identifying RSA.
Keywords: Biomarker; Human decidual mesenchymal stem cells; Recurrent spontaneous abortion; microRNA.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Oliveira M.T.S., Oliveira C.N.T., Marques L.M., Souza C.L., Oliveira M.V. Factors associated with spontaneous abortion: a systematic review. Rev. Bras. Saúde Materno Infant. 2020;20:361–372.
-
- Pandey M.K., Rani R., Agrawal S. An update in recurrent spontaneous abortion. Arch. Gynecol. Obstet. 2005;272:95–108. - PubMed
-
- Branch D.W., Heuser C. Reproductive Endocrinology and Infertility: Integrating Modern Clinical and Laboratory Practice. 2010. Recurrent miscarriage; pp. 281–296.
-
- Rai R., Regan L. Recurrent miscarriage. The lancet. 2006;368(9535):601–611. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

