Spatially resolved transcriptomics reveal the determinants of primary resistance to immunotherapy in NSCLC with mature tertiary lymphoid structures
- PMID: 39909044
- PMCID: PMC11866545
- DOI: 10.1016/j.xcrm.2025.101934
Spatially resolved transcriptomics reveal the determinants of primary resistance to immunotherapy in NSCLC with mature tertiary lymphoid structures
Abstract
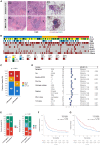
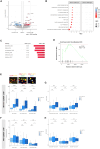
Effectiveness of immune checkpoint inhibitors (ICIs) in non-small cell lung cancer (NSCLC) has been linked to the presence of mature tertiary lymphoid structures (mTLSs) within the tumor microenvironment (TME). However, only a subset of mTLS-positive NSCLC derives benefit, thus highlighting the need to unravel ICI response determinants. The comprehensive analysis of ICI-treated patients with NSCLC (n = 509) from the Bergonié Institute Profiling (BIP) study (NCT02534649) reveals that the presence of mTLSs correlates with improved clinical outcomes, independently of programmed death ligand 1 (PD-L1) expression and genomic features. Employing spatial transcriptomics alongside multiplex immunofluorescence (mIF), we show that two distinct subsets of cancer-associated fibroblasts (CAFs) are essential factors in mediating primary resistance to ICIs in mTLS-positive NSCLC. These CAFs are associated with immune exclusion, CD8+ T cell exhaustion, and increased regulatory CD4+ T cell infiltration, underscoring an immunosuppressive TME. Our study highlights the pivotal role of specific CAF subsets in thwarting ICIs, proposing new therapeutic targets to enhance immunotherapy efficacy.
Keywords: cancer-associated fibroblasts; fibroblasts; immune checkpoint blockade; immune exclusion; immunotherapy; non-small cell lung cancer; regulatory T cells; tertiary lymphoid structures.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests F.P., J.-P.G., C.R., O.L., O.O., and A.B. are employees of Explicyte. R.J.J. is an employee and stockholder of Roche/Genentech. A.I. received research grants from AstraZeneca, Bayer, BMS, Chugai, Merck, MSD, Pharmamar, Novartis, and Roche and personal fees from Epizyme, Bayer, Deciphera, Lilly, Parthenon, Roche, and Springworks.
Figures



References
-
- Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics, 2024. CA. Cancer J. Clin. 2024;74:12–49. - PubMed
-
- Wang M., Herbst R.S., Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat. Med. 2021;27:1345–1356. - PubMed
-
- Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016;375:1823–1833. - PubMed
-
- Gettinger S., Horn L., Jackman D., Spigel D., Antonia S., Hellmann M., Powderly J., Heist R., Sequist L.V., Smith D.C., et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J. Clin. Oncol. 2018;36:1675–1684. - PubMed
-
- Gandhi L., Rodríguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., Domine M., Clingan P., Hochmair M.J., Powell S.F., et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:2078–2092. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

