Pemphigus Vulgaris Autoantibodies Induce an Endoplasmic Reticulum Stress Response
- PMID: 39909113
- PMCID: PMC12317713
- DOI: 10.1016/j.jid.2024.12.028
Pemphigus Vulgaris Autoantibodies Induce an Endoplasmic Reticulum Stress Response
Abstract
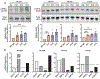
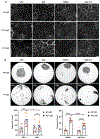
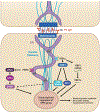
Desmosomes are intercellular junctions that mediate cell-cell adhesion and are essential for maintaining tissue integrity. Pemphigus vulgaris (PV) is an autoimmune epidermal blistering disease caused by autoantibodies (IgG) targeting desmoglein 3, a desmosomal cadherin. PV autoantibodies cause desmosome disassembly and loss of cell-cell adhesion; however, the molecular signaling pathways that regulate these processes are not fully understood. Using high-resolution time-lapse imaging of live keratinocytes, we found that endoplasmic reticulum (ER) tubules make frequent and persistent contacts with internalizing desmoglein 3 puncta in keratinocytes treated with IgG of patients with PV. Biochemical experiments demonstrated that PV IgG activated ER stress signaling pathways, including both IRE1⍺ and PERK pathways, in cultured keratinocytes. Furthermore, ER stress transcripts were upregulated in the skin of patients with PV. Pharmacological inhibition of ER stress protects against PV IgG-induced desmosome disruption and loss of keratinocyte cell-cell adhesion, suggesting that ER stress may be an important pathomechanism and a therapeutically targetable pathway for PV treatment. These data support a model in which desmosome adhesion is integrated with ER function to serve as a cell adhesion stress sensor that is activated in blistering skin diseases.
Keywords: Cadherins; Desmosomes; ER stress; Endoplasmic reticulum; Pemphigus.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
The authors state no conflict of interest.
Figures


Update of
-
Pemphigus vulgaris autoantibodies induce an ER stress response.bioRxiv [Preprint]. 2024 Aug 23:2024.08.22.608849. doi: 10.1101/2024.08.22.608849. bioRxiv. 2024. Update in: J Invest Dermatol. 2025 Sep;145(9):2219-2228.e4. doi: 10.1016/j.jid.2024.12.028. PMID: 39229110 Free PMC article. Updated. Preprint.
References
-
- Abeles I, Palma C, Meednu N, Payne AS, Looney RJ, Anolik JH. B Cell-Directed Therapy in Autoimmunity. Annu Rev Immunol 2024;42(1):103–26. - PubMed
-
- Abian O, Alfonso P, Velazquez-Campoy A, Giraldo P, Pocovi M, Sancho J. Therapeutic strategies for Gaucher disease: miglustat (NB-DNJ) as a pharmacological chaperone for glucocerebrosidase and the different thermostability of velaglucerase alfa and imiglucerase. Mol Pharm 2011;8(6):2390–7. - PubMed
-
- Alfonso P, Pampín S, Estrada J, Rodríguez-Rey JC, Giraldo P, Sancho J, et al. Miglustat (NB-DNJ) works as a chaperone for mutated acid β-glucosidase in cells transfected with several Gaucher disease mutations. Blood Cells, Molecules, and Diseases 2005;35(2):268–76. - PubMed
-
- Angulo-Urarte A, van der Wal T, Huveneers S. Cell-cell junctions as sensors and transducers of mechanical forces. Biochim Biophys Acta Biomembr 2020;1862(9):183316. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

