Mobility of four structural regions drives isoform-specific properties of photoenzyme LPOR in plants
- PMID: 39909377
- PMCID: PMC11930087
- DOI: 10.1016/j.jbc.2025.108261
Mobility of four structural regions drives isoform-specific properties of photoenzyme LPOR in plants
Abstract
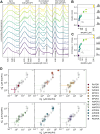
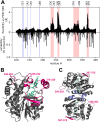
Light-dependent protochlorophyllide oxidoreductase (LPOR) is a photocatalytic enzyme in the chlorophyll biosynthetic pathway that underwent duplications in angiosperms, resulting in the emergence of multiple isoforms across various plant species. The physiological roles of these LPOR homologs remained unclear, so we selected six plant species with different number of isoforms of the enzyme, having diverse phylogenetic backgrounds, and characterized their properties in vitro. Our findings revealed that these isoforms vary in their affinity for the reaction product, chlorophyllide (Chlide), as well as for NADPH under lipid-free conditions and in reaction mixtures supplemented with plant lipids. Additionally, we observed differences in their oligomerization behavior. Our experimental approach generated a dataset comprising several hundred pairs of spectra, recorded before and after reaction-triggering illumination. This data was used to analyze the correlation between fluorescence emission maxima before and after photoconversion. The analysis showed that some isoforms rapidly release Chlide after the reaction, while others retain the pigment in the binding pocket, especially at high NADPH concentrations. These results suggest that LPOR isoforms differ in their rates of Chlide release and complex disassembly, potentially influencing the overall rate of the chlorophyll biosynthetic pathway, even in mature leaves. We further analyzed the flexibility of these isoforms using AlphaFold2 predictions, identifying four regions of the enzyme that are particularly mobile. Two of these regions are involved in pigment binding, while the other two play a role in oligomerization. Based on these findings, we propose a model of conformational changes that drive the formation of LPOR oligomers.
Keywords: in vitro; light-dependent protochlorophyllide oxidoreductase; photoenzyme; photomorphogenesis; recombinant proteins.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures






Similar articles
-
The origin, evolution and diversification of multiple isoforms of light-dependent protochlorophyllide oxidoreductase (LPOR): focus on angiosperms.Biochem J. 2020 Jun 26;477(12):2221-2236. doi: 10.1042/BCJ20200323. Biochem J. 2020. PMID: 32568402
-
Photocatalytic LPOR forms helical lattices that shape membranes for chlorophyll synthesis.Nat Plants. 2021 Apr;7(4):437-444. doi: 10.1038/s41477-021-00885-2. Epub 2021 Apr 19. Nat Plants. 2021. PMID: 33875834
-
Crystal structures of cyanobacterial light-dependent protochlorophyllide oxidoreductase.Proc Natl Acad Sci U S A. 2020 Apr 14;117(15):8455-8461. doi: 10.1073/pnas.1920244117. Epub 2020 Mar 31. Proc Natl Acad Sci U S A. 2020. PMID: 32234783 Free PMC article.
-
Photocatalysis as the 'master switch' of photomorphogenesis in early plant development.Nat Plants. 2021 Mar;7(3):268-276. doi: 10.1038/s41477-021-00866-5. Epub 2021 Mar 8. Nat Plants. 2021. PMID: 33686224 Review.
-
Light dependent protochlorophyllide oxidoreductase: a succinct look.Physiol Mol Biol Plants. 2024 May;30(5):719-731. doi: 10.1007/s12298-024-01454-5. Epub 2024 May 29. Physiol Mol Biol Plants. 2024. PMID: 38846463 Free PMC article. Review.
References
-
- Gabruk M., Mysliwa-Kurdziel B.J. Light-dependent protochlorophyllide oxidoreductase: phylogeny, regulation and catalytic properties. Biochemistry. 2015;54:5255–5262. - PubMed
-
- Solymosi K., Schoefs B. Plant Cell Compartments- Selected Top. Vol. 661. Research Signpost; Trivandrum: 2008. Prolamellar body: a unique plastid compartment, which does not only occur in dark- grown leaves.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

