The effectiveness and risks of Treating people with Idiopathic Pulmonary fibrosis with the Addition of Lansoprazole (TIPAL): study protocol for a randomised placebo-controlled multicentre clinical trial
- PMID: 39909521
- PMCID: PMC11800218
- DOI: 10.1136/bmjopen-2024-088604
The effectiveness and risks of Treating people with Idiopathic Pulmonary fibrosis with the Addition of Lansoprazole (TIPAL): study protocol for a randomised placebo-controlled multicentre clinical trial
Abstract
Introduction: Idiopathic pulmonary fibrosis (IPF) is a chronic progressive fibrotic lung disease frequently complicated by gastro-oesophageal reflux disease. Although several observational studies and a pilot study have investigated the role of proton pump inhibitors (PPIs) in IPF, their efficacy is unknown and there is much debate in international IPF guidelines on their use. We aim to undertake an adequately powered double-blind placebo-controlled randomised multicentre clinical trial to assess the change in forced vital capacity (FVC), cough and other important patient-reported outcomes, following 12-month therapy with PPIs in people with IPF.
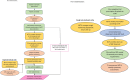
Methods and analysis: A total of 298 patients with IPF diagnosed by a multidisciplinary team according to international guidelines who are not receiving PPIs will be enrolled. Patients are randomised equally to receive two capsules of lansoprazole or two placebo capsules, two times per day for 12 months. The primary outcome for the trial is change in FVC, measured at home, between the first week and last week of the study period. Secondary assessments include cough frequency (in a subgroup) measured using the VitaloJAK cough monitor, the King's Brief Interstitial Lung Disease questionnaire, the Raghu Scale for Pulmonary Fibrosis, Medical Research Council dyspnoea score, EQ-5D-5L, Leicester Cough Questionnaire, modified DeMeester reflux symptoms questionnaire and opportunistically captured routine lung function measurements. High-resolution CT scoring will be undertaken in a subgroup. The trial is designed to determine whether treating people with IPF with lansoprazole will reduce the reduction in FVC over a year. The COVID-19 pandemic required the study to be undertaken as a remote trial.
Ethics and dissemination: This study received ethical approval from the East of England Cambridgeshire and Hertfordshire Research Ethics Committee (reference 20/EE/0043; integrated research application system number 269050). Trial results will be published in a peer-reviewed journal upon completion.
Trial registration number: ISRCTN13526307; ClinicalTrials.gov NCT04965298.
Keywords: Clinical Trial; Computed tomography; Pulmonary Disease.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Cyclophosphamide for connective tissue disease-associated interstitial lung disease.Cochrane Database Syst Rev. 2018 Jan 3;1(1):CD010908. doi: 10.1002/14651858.CD010908.pub2. Cochrane Database Syst Rev. 2018. PMID: 29297205 Free PMC article.
-
Jinbei oral liquid for idiopathic pulmonary fibrosis: a randomized placebo-controlled trial.Sci Rep. 2025 Jan 23;15(1):3007. doi: 10.1038/s41598-025-87474-x. Sci Rep. 2025. PMID: 39849152 Free PMC article. Clinical Trial.
-
Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD004823. doi: 10.1002/14651858.CD004823.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2006 Oct 18;(4):CD004823. doi: 10.1002/14651858.CD004823.pub3. PMID: 15846735 Updated.
-
Early nintedanib deployment in COVID-19 interstitial lung disease (ENDCOV-I): study protocol of a randomised, double-blind, placebo-controlled trial.BMJ Open Respir Res. 2025 Apr 10;12(1):e002323. doi: 10.1136/bmjresp-2024-002323. BMJ Open Respir Res. 2025. PMID: 40216412 Free PMC article.
-
Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults.Cochrane Database Syst Rev. 2006 Oct 18;(4):CD004823. doi: 10.1002/14651858.CD004823.pub3. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2011 Jan 19;(1):CD004823. doi: 10.1002/14651858.CD004823.pub4. PMID: 17054216 Updated.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
