HER-SAFE study design: an open-label, randomised controlled trial to investigate the safety of withdrawal of pharmacological treatment for recovered HER2-targeted therapy-related cardiac dysfunction
- PMID: 39909533
- PMCID: PMC11800297
- DOI: 10.1136/bmjopen-2024-091917
HER-SAFE study design: an open-label, randomised controlled trial to investigate the safety of withdrawal of pharmacological treatment for recovered HER2-targeted therapy-related cardiac dysfunction
Abstract
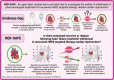
Introduction: A quarter of breast cancers show human epidermal growth factor-2 (HER2) overexpression, where targeted therapy dramatically improves survival. However, cancer therapy-related cardiac dysfunction (CTRCD) occurs in up to 15% of patients. With the interruption of HER2 therapy, if necessary, and the initiation of heart failure therapy (HFT), HER2 CTRCD recovers in over 80% of cases. The need to continue HFT in 'recovered' HER2 CTRCD following completion of HER2 therapy is unclear and there are potential significant impacts on patient's quality of life (QoL). The Randomised Controlled Trial for the Safety of Withdrawal of Pharmacological Treatment for Recovered HER2 Targeted Therapy Related Cardiac Dysfunction (HER-SAFE) aims to evaluate whether HFT can be safely withdrawn in non-high cardiovascular (CV) risk patients with 'recovered' HER2 CTRCD.
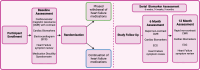
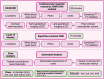
Methods and analysis: This is a multicentre, open-label randomised controlled trial investigating whether withdrawal of HFT is non-inferior to continuation in non-high CV risk, breast cancer survivors with recovered HER2 CTRCD after cancer treatment completion. The primary endpoint is the incidence of guideline-defined cardiac dysfunction or clinical heart failure. Secondary endpoints include changes in cardiac blood biomarkers, cardiovascular magnetic resonance (CMR)-derived strain and tissue mapping and heart failure symptom questionnaires. The study will recruit 90 participants who will undergo serial clinical assessment over 12 months with advanced cardiovascular imaging (CMR scans with automated analysis at baseline, 6 and 12 months), cardiac biomarker measurement (six time points over 12 months), plus complete heart failure QoL and medication disutility questionnaires. This is the first multicentre study to address this significant clinical issue.
Ethics and dissemination: This study was approved by the research ethics committee (London-London Bridge, 23/LO/0152). The results will be disseminated in peer-reviewed scientific journals.
Trial registration number: NCT05880160.
Keywords: Breast tumours; Cardiovascular Disease; Cardiovascular imaging; Heart failure.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: CHM is cofounder of MyCardium AI and has received speaker honoraria from Pfizer, BeiGene, Bayer, Abbott Medical and Biotronik. ARL has received speaker, advisory board or consultancy fees and/or research grants from Pfizer, Novartis, Servier, AstraZeneca, Bristol Myers Squibb, GSK, Amgen, Takeda, Roche, Janssen-Cilag, Clinigen Group, Eli Lily, Eisai, Ferring Pharmaceuticals, Boehringer Ingelheim, Akcea Therapeutics, Myocardial Solutions, iOWNA Health, Heartfelt Technologies, ARMA Biosciences and EpicLife. All other authors have no completing interest to declare.
Figures

References
-
- Breast cancer statistics World cancer research fund international. [29-Apr-2024]. https://www.wcrf.org/cancer-trends/breast-cancer-statistics/ Available. Accessed.
-
- Cancer Registration Statistics England 2019 - NHS digital. [16-Jan-2022]. https://digital.nhs.uk/data-and-information/publications/statistical/can... Available. Accessed.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
