Survival and quality of life in patients with lower risk myelodysplastic syndromes exposed to erythropoiesis-stimulating agents: an observational cohort study
- PMID: 39909656
- PMCID: PMC11803517
- DOI: 10.1016/S2352-3026(24)00350-8
Survival and quality of life in patients with lower risk myelodysplastic syndromes exposed to erythropoiesis-stimulating agents: an observational cohort study
Erratum in
-
Correction to Lancet Haematol 2025; 12: e128-37.Lancet Haematol. 2025 Apr;12(4):e243. doi: 10.1016/S2352-3026(25)00076-6. Lancet Haematol. 2025. PMID: 40174998 Free PMC article. No abstract available.
Abstract
Background: In our previous study on erythropoiesis-stimulating agent (ESA) treatment in lower risk myelodysplastic syndromes from the European MDS (EUMDS) Registry, we showed that patients treated with ESAs had longer survival compared with patients who receive red blood cell transfusion (RBCT). In this study, with a longer follow up time and more patients included, we aimed to assess long-term effects on survival and health-related quality of life (HRQoL) of exposure to ESAs with or without RBCT in patients with lower risk myelodysplastic syndromes.
Methods: The EUMDS Registry is a non-interventional, longitudinal, real-world registry prospectively enrolling newly diagnosed patients older than 18 years with lower risk (International Prognostic Scoring System low or intermediate-1) myelodysplastic syndromes from 16 European countries and Israel. The analysis was restricted to patients with haemoglobin concentrations less than 100 g/L enrolled between Jan 1, 2008, and July 1, 2019, with last censoring of data on Dec 31, 2021. Patient management was recorded every 6 months, including treatment, transfusions, and HRQoL. ESA treatment followed local guidelines. The patients were separated into four groups at each study visit: no ESA or RBCT, ESA only, ESA plus RBCT, and RBCT only. The data were analysed longitudinally over time according to ESA and RBCT status during each 6-month interval, using propensity score matching. The main outcomes were median overall survival and leukaemia-free survival, and HRQoL. This study is registered with ClinicalTrials.gov, NCT00600860, as is ongoing.
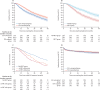
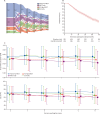
Findings: 2448 patients (the ESA-unexposed group [n=1265] and ESA-exposed group [n=1183]) were diagnosed before July 1, 2019; 1520 (62·1%) were male and 928 (37·9%) were female. Median follow-up time was 3·9 years (IQR 1·6-6·5). After applying eligibility criteria and propensity matching, there were 426 patients in the ESA-unexposed group and 744 patients in the ESA-exposed group. Median overall survival in the ESA exposed group was 44·9 months (95% CI 40·2-50·5) compared with 34·8 months (28·6-39·2) in the ESA unexposed group; the absolute difference was 10·1 months (95% CI 2·2-18·0; hazard ratio [HR] 0·70 [95% CI 0·59-0·83]; p<0·0001). Patients without RBCT in the presence or absence of ESA exposure maintained significantly better HRQoL than those with RBCT, irrespective of ESA exposure (linear mixed effect model of EQ-5d-3L index score, RBCT coefficient -0·04 [95% CI -0·06 to 0·03], p<0·0001; linear mixed effect model of VAS, -4·57 [-6·02 to -3·13], p<0·0001).
Interpretation: ESA treatment in patients with lower risk myelodysplastic syndromes significantly improves overall survival when started before or early after the onset of regular transfusion therapy. Avoiding RBCT is associated with significantly better HRQoL.
Funding: H2020 European Research Council, Novartis Pharmacy B V Oncology Europe, Amgen, BMS/Celgene International, Janssen Pharmaceutica, Takeda Pharmaceuticals International, and Gilead Sciences.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests RS reports honoraria from BMS for the MDS Right Horizon 2020 Project; financial support for meetings from Celgene/BMS, Lilly, Teva, AbbVie, and AHOP; advisory board participation for BMS and Otsuka; and is Servier President of Verein Senioren Krebshilfe. CvM and TdW work for the EUMDS Registry, which is supported by an educational grant from Novartis Pharmacy BV Oncology Europe, Amgen, BMS, Janssen Pharmaceutical, Takeda Pharmaceutical, Gilead Sciences, and a European commission H2020 Grant. AS is supported in part by the National Institute for Health and Care Research (NIHR) Leeds Biomedical Research Centre (NIHR203331). HKGG, TB, AT, PF, DB, ArS, JC, SL, LM, UG, LS, MdA, DC, KAK, SCr, and EHL declare no competing interests.
Figures
Comment in
-
Improved survival and enhanced quality of life through anaemia correction in lower risk myelodysplastic syndromes: meaningful insights from an EUMDS Registry study.Lancet Haematol. 2025 Feb;12(2):e88-e90. doi: 10.1016/S2352-3026(24)00355-7. Lancet Haematol. 2025. PMID: 39909660 No abstract available.
References
-
- Greenberg PL, Stone RM, Al-Kali A, et al. NCCN Guidelines® Insights: Myelodysplastic Syndromes, Version 3.2022. J Natl Compr Canc Netw. 2022;20:106–117. - PubMed
-
- Olsnes Kittang A. Guidelines for the diagnosis and treatment of myelodysplastic syndrome and chronic myelomonocytic leukemia. 2021. https://www.nmds.org/media/4/download
-
- Gascón P, Krendyukov A, Mathieson N, Aapro M. Epoetin alfa for the treatment of myelodysplastic syndrome-related anemia: a review of clinical data, clinical guidelines, and treatment protocols. Leuk Res. 2019;81:35–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

