Role of brain-derived neurotrophic factor in dysfunction of short-term to long-term memory transformation after surgery and anaesthesia in older mice
- PMID: 39909796
- PMCID: PMC11947570
- DOI: 10.1016/j.bja.2024.11.045
Role of brain-derived neurotrophic factor in dysfunction of short-term to long-term memory transformation after surgery and anaesthesia in older mice
Abstract
Background: Memory decline is one of the main manifestations in perioperative neurocognitive disorder. Short-term memory (STM) to long-term memory (LTM) transformation is one aspect of memory consolidation. Early-phase long-term potentiation (E-LTP) to late-phase long-term potentiation (L-LTP) is the molecular correlate of STM to LTM transformation. We examined whether the STM to LTM transformation was impaired after anaesthesia and surgery in older mice.
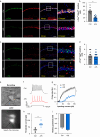
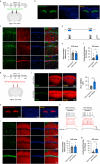
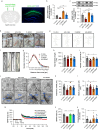
Methods: Optogenetics and chemogenetics were used to confirm the role of Vglut1+ glutamatergic neurones in the STM to LTM transformation in older mice. Synaptosomes were isolated to analyse expression of brain-derived neurotrophic factor (BDNF). Golgi-Cox staining and hippocampal field potential recordings were also used to measure synaptic plasticity.
Results: We found that the STM to LTM and E-LTP to L-LTP transformations were impaired after anaesthesia and surgery in older mice, and Vglut1+ excitatory neurone activity in the hippocampal CA1 region was reduced. BDNF expression decreased in the postsynaptic fraction, especially in Vglut1+ neurones, whereas cell-type specific overexpression of BDNF in Vglut1+ neurones reversed postoperative STM to LTM transformation dysfunction in older mice.
Conclusions: Reduced BDNF expression was involved in anaesthesia and surgery-induced impairment of the STM to LTM transition involving glutamatergic neurones in the hippocampal CA1 region of older mice. This provides a potential target that might be helpful for understanding and developing treatments for postoperative neurocognitive dysfunction.
Keywords: behavioural tagging; brain-derived neurotrophic factor; memory; perioperative neurocognitive dysfunction; synaptic tagging.
Copyright © 2025 British Journal of Anaesthesia. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interest The authors declare that they have no conflict of interest.
Figures



References
-
- Borchers F., Spies C.D., Feinkohl I., et al. Methodology of measuring postoperative cognitive dysfunction: a systematic review. Br J Anaesth. 2021;126:1119–1127. - PubMed
-
- Sungur Z., Şentürk M. Might complications of analgesic regimen affect cognitive assessment and how to diagnose POCD? Reg Anesth Pain Med. 2020;45:938–939. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

