Aldh2 and the tumor suppressor Trp53 play important roles in alcohol-induced squamous field cancerization
- PMID: 39909947
- PMCID: PMC12014750
- DOI: 10.1007/s00535-024-02210-y
Aldh2 and the tumor suppressor Trp53 play important roles in alcohol-induced squamous field cancerization
Abstract
Background: Field cancerization defined by multiple development of squamous cell carcinomas (SCCs) in upper aerodigestive tract was explained by excessive alcohol intake. A dysfunctional mitochondrial aldehyde dehydrogenase 2 (Aldh2) delays the clearance of acetaldehyde, a genotoxic alcohol metabolite, and increases SCC risks. TP53 plays key roles in squamous carcinogenesis. However, the mechanism of alcohol-mediated squamous field cancerization has not been clearly elucidated.
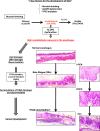
Methods: We developed a novel genetically engineered mouse strain KTPA-/- (Krt5CreERT2; Trp53loxp/loxp; Aldh2-/-) featuring Aldh2-loss concurrent with epithelial-specific Trp53 deletion. These mice were given 10%-EtOH, and we evaluated the development of squamous cell carcinogenesis histologically and genetically.
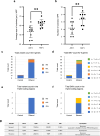
Results: Widespread multifocal rete ridges (RRs), characterized by downward growth of proliferative preneoplastic cells, were found only in Aldh2+/- and Aldh2-/- mice with keratin5-specific Trp53 deletion (KTPA+/- and KTPA-/- mice, respectively), and alcohol drinking apparently increased RR formation rate. SCC occurred only in KTPA-/- (Aldh2 loss/TP53 loss) mice with alcohol drinking (15/18: 83%). Total alcohol consumption volume was significantly higher in KTPA-/- (Aldh2 loss/TP53 loss) mice with SCCs than those without SCCs. Further, target sequence revealed the occurrence of genetic abnormalities including Trp53 mutations in the esophageal epithelium of Aldh2-/- mice with alcohol drinking, suggesting direct mutagenic effects of alcohol drinking to the esophageal epithelium.
Conclusion: This study provides for the first time the evidence that alcohol drinking, Aldh2 dysfunction and Trp53 loss cooperate in squamous field cancerization. Alcohol consumption volume affects the SCCs development, even in the same genotype.
Keywords: Alcohol-drinking; Aldh2; Field cancerization; SCC; TP53.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest statement: All authors declare that they have no competing interests.
Figures




References
-
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–8. - PubMed
-
- Muto M, Hironaka S, Nakane M, et al. Association of multiple Lugol-voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest Endosc. 2002;56:517–21. - PubMed
-
- Baan R, Straif K, Grosse Y, et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8:292–3. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

