Perioperative tislelizumab plus chemotherapy for locally advanced gastroesophageal junction adenocarcinoma (NEOSUMMIT-03): a prospective, nonrandomized, open-label, phase 2 trial
- PMID: 39910052
- PMCID: PMC11799164
- DOI: 10.1038/s41392-025-02160-8
Perioperative tislelizumab plus chemotherapy for locally advanced gastroesophageal junction adenocarcinoma (NEOSUMMIT-03): a prospective, nonrandomized, open-label, phase 2 trial
Abstract
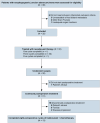
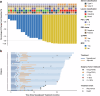
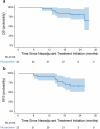
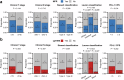
This prospective, nonrandomized, open-label phase 2 trial (Chinese Clinical Trial Registry, ChiCTR2200061906) aimed to evaluate the effectiveness of adding the PD-1 antibody tislelizumab to perioperative chemotherapy in patients with locally advanced gastroesophageal junction adenocarcinoma (GEJA). This study enrolled patients with GEJA clinically staged as cT3-4aNanyM0 or cT1-2N+M0 from October 2022 to June 2023. Eligible patients were administered three preoperative and five postoperative 3-week cycles of treatment with PD-1 antibody tislelizumab plus SOX (S-1 and oxaliplatin) regimen. The primary endpoint was major pathological response (MPR) rate. Thirty-two patients were enrolled. The median age was 60 years (range: 28-74 years), and 53.1% (17/32) patients were Siewert III type. All patients received at least one cycle of assigned preoperative treatment, and 93.8% (30/32) patients completed three cycles of assigned preoperative tislelizumab and SOX. The R0 resection rate was 96.9% (31/32). MPR, pathological complete response (pCR) of primary tumors and ypT0N0 rates were 50.0% (16/32, 95% CI: 31.9-68.1%), 28.1% (9/32, 95% CI: 13.7-46.7%) and 25.0% (8/32, 95% CI: 11.5-43.4%), respectively. The surgical morbidity rate was 15.6% (5/32), and no 30-day mortality was observed. In the preoperative and postoperative treatment periods, the rate of treatment-related grade 3-4 adverse events was 31.2% (10/32). At the date of 7th Jan 2025, 8 (25.0%) patients occurred recurrence. Therefore, perioperative tislelizumab plus chemotherapy demonstrated significantly improved pathological regression and might be a promising option for patients with locally advanced resectable GEJA.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74, 229–263 (2024). - PubMed
-
- Arnold, M., Ferlay, J., van Berge Henegouwen, M. I. & Soerjomataram, I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut69, 1564–1571 (2020). - PubMed
-
- Nie, R. C. et al. Adjuvant chemotherapy for patients with adenocarcinoma of the esophagogastric junction: a retrospective, multicenter, observational study. Ann. Surg. Oncol.30, 4014–4025 (2023). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

