An oligodendrocyte silencer element underlies the pathogenic impact of lamin B1 structural variants
- PMID: 39910058
- PMCID: PMC11799162
- DOI: 10.1038/s41467-025-56378-9
An oligodendrocyte silencer element underlies the pathogenic impact of lamin B1 structural variants
Abstract
The role of non-coding regulatory elements and how they might contribute to tissue type specificity of disease phenotypes is poorly understood. Autosomal Dominant Leukodystrophy (ADLD) is a fatal, adult-onset, neurological disorder that is characterized by extensive CNS demyelination. Most cases of ADLD are caused by tandem genomic duplications involving the lamin B1 gene (LMNB1) while a small subset are caused by genomic deletions upstream of the gene. Utilizing data from recently identified families that carry LMNB1 gene duplications but do not exhibit demyelination, ADLD patient tissues, CRISPR edited cell lines and mouse models, we have identified a silencer element that is lost in ADLD patients and that specifically targets expression to oligodendrocytes. This element consists of CTCF binding sites that mediate three-dimensional chromatin looping involving LMNB1 and the recruitment of the PRC2 transcriptional repressor complex. Loss of the silencer element in ADLD identifies a role for non-coding regulatory elements in tissue specificity and disease causation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests
Figures




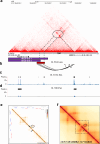



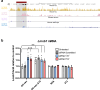
Update of
-
An oligodendrocyte silencer element underlies the pathogenic impact of lamin B1 structural variants.bioRxiv [Preprint]. 2023 Aug 9:2023.08.03.551473. doi: 10.1101/2023.08.03.551473. bioRxiv. 2023. Update in: Nat Commun. 2025 Feb 05;16(1):1373. doi: 10.1038/s41467-025-56378-9. PMID: 37609196 Free PMC article. Updated. Preprint.
References
-
- Raininko, R., Gosky, M.& Padiath, Q. S. LMNB1-related autosomal dominant leukodystrophy. In: GeneReviews((R)) (eds Adam MP, et al.) (2021). - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 NS126193/NS/NINDS NIH HHS/United States
- R21 NS131906/NS/NINDS NIH HHS/United States
- S10 OD028483/OD/NIH HHS/United States
- R21 AG046897/AG/NIA NIH HHS/United States
- R01 NS095884/NS/NINDS NIH HHS/United States
- R33 NS104384/NS/NINDS NIH HHS/United States
- R35 GM133732/GM/NIGMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- DP2 GM146336/GM/NIGMS NIH HHS/United States
- R01NS126193, R21NS131906, R33NS104384, R33NS106087, R01NS095884/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R33 NS106087/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

