Neurovascularization inhibiting dual responsive hydrogel for alleviating the progression of osteoarthritis
- PMID: 39910066
- PMCID: PMC11799281
- DOI: 10.1038/s41467-025-56727-8
Neurovascularization inhibiting dual responsive hydrogel for alleviating the progression of osteoarthritis
Abstract
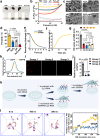
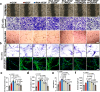
Treating osteoarthritis (OA) associated pain is a challenge with the potential to significantly improve patients lives. Here, we report on a hydrogel for extracellular RNA scavenging and releasing bevacizumab to block neurovascularization at the osteochondral interface, thereby mitigating OA pain and disease progression. The hydrogel is formed by cross-linking aldehyde-phenylboronic acid-modified sodium alginate/polyethyleneimine-grafted protocatechuic acid (OSAP/PPCA) and bevacizumab sustained-release nanoparticles (BGN@Be), termed OSPPB. The dynamic Schiff base bonds and boronic ester bonds allow for injectability, self-healing, and pH/reactive oxygen species dual responsiveness. The OSPPB hydrogel can significantly inhibit angiogenesis and neurogenesis in vitro. In an in vivo OA model, intraarticular injection of OSPPB accelerates the healing process of condyles and alleviates chronic pain by inhibiting neurovascularization at the osteochondral interface. The injectable hydrogel represents a promising technique to treat OA and OA associated pain.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

