Impact of low muscle mass and myosteatosis on treatment toxicity and survival outcomes in non-resectable pancreatic cancer patients treated with chemoradiotherapy
- PMID: 39910182
- PMCID: PMC12151844
- DOI: 10.1038/s41430-025-01566-5
Impact of low muscle mass and myosteatosis on treatment toxicity and survival outcomes in non-resectable pancreatic cancer patients treated with chemoradiotherapy
Abstract
Background: Low skeletal muscle mass and impaired muscle quality (myosteatosis) have been associated with poor outcomes in cancer patients. This study aimed to evaluate the impact of pre-therapeutic low muscle mass and myosteatosis on chemoradiotherapy (CRT)-induced toxicity and survival outcomes in patients with non-resectable pancreatic cancer (PC).
Methods: In this retrospective study, pre-therapeutic CT scans were used to measure muscle mass/density. Low muscle mass was defined as a skeletal muscle index <38.5 cm²/m² (women) and <52.4 cm²/m² (men), and myosteatosis as a mean psoas density <41 HU if BMI < 25 kg/m² or <33 HU if BMI > 25 kg/m². Adverse effects were collected per week (W) of treatment. Dose-limiting toxicity (DLT) was defined as any toxicity leading to dose reduction, treatment delays or permanent discontinuation.
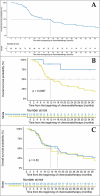
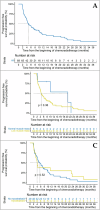
Results: Among the 85 included patients, 75 (88.2%) and 18 (22.2%) had pre-therapeutic low muscle mass and myosteatosis, respectively. Only 12 patients (14.1%) experienced DLT. Patients with low muscle mass developed significantly more toxicities at W2 (p = 0.013) and W5 (p = 0.026), notably more nausea (p = 0.037) and anemia (p = 0.004). Low muscle mass was associated with poorer overall survival (HR 4.41 [1.50-12.94], p = 0.007) in multivariate Cox analysis, while myosteatosis was not associated with CRT toxicities, DLT and overall survival (p = 0.408).
Conclusion: Patients with low muscle mass experienced more toxicities and poorer outcomes during CRT for non-resectable PC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: CC reports personal fees as a speaker from Brystol Myers Squibb, outside the submitted work. DB reports personal fees as a speaker and/or in an advisory role from Accord Healthcare, Amgen, Sanofi, Servier, and Pierre Fabre, outside the submitted work. FS reports personal fees as a speaker and/or in an advisory role from Astra Zeneca, outside the submitted work. OB reports personal fees as a speaker and/or in an advisory role from Merck KGaA, Apmomia Therapeutics, Bayer, MSD, Amgen, Servier, and Pierre Fabre, outside the submitted work. All other authors have no conflict of interest. Ethical approval: The database of this retrospective study was constituted in accordance with the reference methodology MR004 of the French National Commission on Informatics and Liberty (CNIL) and the Helsinki Declaration. According to current French regulations, no informed consent or additional ethical committee review was required for retrospective studies using data from medical records collected in daily practice by medical and nursing staff caring for the patient and without human involvement. Data were anonymized prior to statistical analysis. Based on the non-opposition principle, information on the purpose of the study and the type of data collected was given to each patient included in the study. Patients were free to decline to participate, but none refused. The present study was declared to the Health Data Hub under the number F20220624135714 and is available at https://www.health-data-hub.fr .
Figures


References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:1028–61. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

