Daratumumab plus bortezomib, lenalidomide and dexamethasone for transplant-ineligible or transplant-deferred newly diagnosed multiple myeloma: the randomized phase 3 CEPHEUS trial
- PMID: 39910273
- PMCID: PMC12003169
- DOI: 10.1038/s41591-024-03485-7
Daratumumab plus bortezomib, lenalidomide and dexamethasone for transplant-ineligible or transplant-deferred newly diagnosed multiple myeloma: the randomized phase 3 CEPHEUS trial
Erratum in
-
Author Correction: Daratumumab plus bortezomib, lenalidomide and dexamethasone for transplant-ineligible or transplant-deferred newly diagnosed multiple myeloma: the randomized phase 3 CEPHEUS trial.Nat Med. 2025 Apr;31(4):1366. doi: 10.1038/s41591-025-03581-2. Nat Med. 2025. PMID: 39948407 Free PMC article. No abstract available.
Abstract
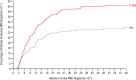
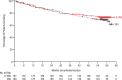
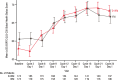
Frontline daratumumab-based triplet and quadruplet standard-of-care regimens have demonstrated improved survival outcomes in newly diagnosed multiple myeloma (NDMM). For patients with transplant-ineligible NDMM, triplet therapy with either daratumumab plus lenalidomide and dexamethasone (D-Rd) or bortezomib, lenalidomide and dexamethasone (VRd) is the current standard of care. This phase 3 trial evaluated subcutaneous daratumumab plus VRd (D-VRd) in patients with transplant-ineligible NDMM or for whom transplant was not planned as the initial therapy (transplant deferred). Some 395 patients with transplant-ineligible or transplant-deferred NDMM were randomly assigned to eight cycles of D-VRd or VRd followed by D-Rd or Rd until progression. The primary endpoint was overall minimal residual disease (MRD)-negativity rate at 10-5 by next-generation sequencing. Major secondary endpoints included complete response (CR) or better (≥CR) rate, progression-free survival and sustained MRD-negativity rate at 10-5. At a median follow-up of 58.7 months, the MRD-negativity rate was 60.9% with D-VRd versus 39.4% with VRd (odds ratio, 2.37; 95% confidence interval (CI), 1.58-3.55; P < 0.0001). Rates of ≥CR (81.2% versus 61.6%; P < 0.0001) and sustained MRD negativity (≥12 months; 48.7% versus 26.3%; P < 0.0001) were significantly higher with D-VRd versus VRd. Risk of progression or death was 43% lower for D-VRd versus VRd (hazard ratio, 0.57; 95% CI, 0.41-0.79; P = 0.0005). Adverse events were consistent with the known safety profiles for daratumumab and VRd. Combining daratumumab with VRd produced deeper and more durable MRD responses versus VRd alone. The present study supports D-VRd quadruplet therapy as a new standard of care for transplant-ineligible or transplant-deferred NDMM. ClinicalTrials.gov registration: NCT03652064 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.Z.U. received research funding from Amgen, Array BioPharma, Bristol Myers Squibb, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDx and Takeda and consulted for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Edo Pharma, Genentech, Gilead, GSK, Janssen, Oncopeptides, Sanofi, Seattle Genetics, Secura Bio, SkylineDx, Takeda and TeneoBio. V.H. received honoraria for lectures/advisory boards from AbbVie, Amgen, Bristol Myers Squibb, GSK, Johnson & Johnson, Pfizer, Regeneron, Sanofi and Takeda. N.J.B. consulted for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Janssen, GSK, Genentech, Karyopharm Therapeutics, Kite, Novartis, Pfizer, Roche, Sanofi and Takeda, received research funding from Janssen and Pfizer and honoraria from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Janssen, GSK, Genentech, Karyopharm Therapeutics, Kite, Novartis, Pfizer, Roche, Sanofi and Takeda and served on the Board of Directors or advisory committees for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Janssen, GSK, Genentech, Karyopharm Therapeutics, Kite, Novartis, Pfizer, Roche, Sanofi and Takeda. C.P.V. received honoraria from Janssen, Bristol Myers Squibb, GSK, Sanofi, Pfizer, AbbVie and Forus. M. Braunstein participated in speakers bureaus for Bristol Myers Squibb, Janssen, Takeda and Sanofi. J.M.M. served as a consultant for Bristol Myers Squibb, Medison Pharma, Pfizer and Roche. Y.C.C. served as a consultant for, received research funding and honoraria from and served on the Board of Directors or advisory committees for Bristol Myers Squibb, Janssen, Takeda, Sanofi and GSK. M.M. received honoraria from Janssen Pharmaceuticals, Ono Pharmaceutical, Takeda Pharmaceuticals, Sanofi K.K. Nippon Kayaku and SymBio Pharmaceuticals and received research funding from Janssen Pharmaceuticals, Bristol Myers Squibb K.K., GSK and Pfizer. K.S. received lecture fees from Takeda, Ono Pharmaceutical, Novartis, Sanofi, Bristol Myers Squibb and Janssen and received advisory fees from SRL. M. Beksac served as a consultant for Bristol Myers Squibb, Takeda, Janssen, Menarini, Amgen and GSK and participated in speakers bureaus for Bristol Myers Squibb, Janssen, Takeda and Sanofi. A.M. served as a consultant for Janssen, Takeda, Amgen, Bristol Myers Squibb, Sanofi, Novartis, AstraZeneca, Pfizer and AbbVie and received honoraria from Janssen, Takeda, Amgen, Bristol Myers Squibb, Sanofi, Novartis, AstraZeneca, Pfizer and AbbVie. H.T. served as a consultant for SRL, received honoraria from Janssen, Ono Pharmaceutical, Sanofi and Bristol Myers Squibb and received research funding from Bristol Myers Squibb. A.P. received research funding from Bristol Myers Squibb, Sanofi and Takeda. T.A. is an employee of Genmab and owns stock. W. Liu, J.W., K.C., J.V., M.K., L.L.-M., J.C., M.R. and R.C. are employees of Janssen. S.Z. received research funding from Janssen and Takeda and participated in advisory boards (fees to institute) for Janssen, Bristol Myers Squibb, Sanofi, Oncopeptides, Amgen and Takeda. The other authors declare no competing interests.
Figures







References
-
- de Weers, M. et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J. Immunol.186, 1840–1848 (2011). - PubMed
-
- Lammerts van Bueren, J. et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood124, 3474 (2014).
-
- Overdijk, M. B. et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J. Immunol.197, 807–813 (2016). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

