H-bonded organic frameworks as ultrasound-programmable delivery platform
- PMID: 39910310
- PMCID: PMC12038167
- DOI: 10.1038/s41586-024-08401-0
H-bonded organic frameworks as ultrasound-programmable delivery platform
Abstract
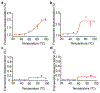
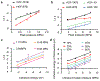
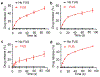
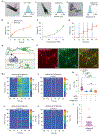
The precise control of mechanochemical activation within deep tissues using non-invasive ultrasound holds profound implications for advancing our understanding of fundamental biomedical sciences and revolutionizing disease treatments1-4. However, a theory-guided mechanoresponsive materials system with well-defined ultrasound activation has yet to be explored5,6. Here we present the concept of using porous hydrogen-bonded organic frameworks (HOFs) as toolkits for focused ultrasound (FUS) programmably triggered drug activation to control specific cellular events in the deep brain, through on-demand scission of the supramolecular interactions. A theoretical model is developed to potentially visualize the mechanochemical scission and ultrasound mechanics, providing valuable guidelines for the rational design of mechanoresponsive materials to achieve programmable control. To demonstrate the practicality of this approach, we encapsulate the designer drug clozapine N-oxide (CNO) into the optimal HOF nanocrystals for FUS-gated release to activate engineered G-protein-coupled receptors in the ventral tegmental area (VTA) of mice and rats and hence achieve targeted neural circuit modulation even at depth 9 mm with a latency of seconds. This work demonstrates the capability of ultrasound to precisely control molecular interactions and develops ultrasound-programmable HOFs to non-invasively and spatiotemporally control cellular events, thereby facilitating the establishment of precise molecular therapeutic possibilities.
© 2025. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: H.W., W.W., Y.S. and B.C. declare that a patent application (PCT/US2024/042314) relating to this work has been filed. The other authors declare no competing interests.
Figures
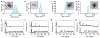


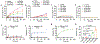


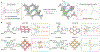



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

