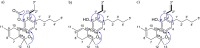Identification of Three Novel Tetrahydrocannabinol Analogs in the European Market
- PMID: 39911051
- PMCID: PMC12401631
- DOI: 10.1002/dta.3866
Identification of Three Novel Tetrahydrocannabinol Analogs in the European Market
Abstract
Synthetic cannabinoids, known as Spice or K2, emerged in Europe and the United States between 2005 and 2008, peaking in incidents by 2015 with severe health implications. In 2021, the identification of hexahydrocannabinol (HHC), a semisynthetic cannabinoid (SSC), led to regulatory control in more than 20 countries in Europe, several US states, and other jurisdictions. A 2024 study in the United States highlighted the diversity of semisynthetic cannabinoids in the US market. New entries of SSCs are increasingly available in the European market, often found as blends in consumer products. This highlights the growing complexity of their regulation and the potential public health risks due to limited toxicological data. The major ingredients, isolated from "CB9", "tresconol", and "CBx", were subjected to mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy. The major isolated ingredient of "CB9" was identified as [2-(E)-propen-1-yl]-Δ8-tetrahydrocannabinol-acetate. The major ingredient of "tresconol" was identified as [2-propen-2-yl]-Δ9-tetrahydrocannabinol, and the major ingredient of "CBx" was identified as [2-propen-2-yl]-Δ8-tetrahydrocannabinol. These compounds have no available spectroscopic or chromatographic data, have never been identified in Cannabis plants, and cannot be identified by standard chromatographic forensic analytical methods, without the use of spectroscopic techniques due to the lack of reference standards. The products were complex mixtures of previously unknown synthetic cannabinoids lacking established safety profiles. These findings highlight the potential public health risks associated with unregulated SSCs, similar to the concerns raised during the 2015 Spice outbreak. The presence of these novel substances requires careful monitoring to prevent future health crises.
Keywords: CB9; CBx; Cannabis public health; semisynthetic cannabinoids; tresconol.
© 2025 The Author(s). Drug Testing and Analysis published by John Wiley & Sons Ltd.
Conflict of interest statement
V. M. is the owner of Ekati Alchemy Lab (Spain), no competing financial interests exist.
The authors declare no conflicts of interest.
Figures
References
-
- Poison Information and Prevention Education Repository , https://piper.filecamp.com/s/i/jbbjDA8ugqAZKl6C/s/v7pJtn8rpZegvPf6.
-
- Center for Disease Control and Prevention , “Synthetic Cannabinoids (Spice): An Overview for Healthcare Providers,” https://archive.cdc.gov/www_cdc_gov/nceh/hsb/envepi/outbreaks/sc/healthc..., (2023).
-
- European Union Drugs Agency , “New Cannabinoid HHC in the Spotlight as Market Evolves,” https://www.euda.europa.eu/news/2023/new‐cannabinoid‐hhc‐spotlight‐marke....
-
- Adams R., Pease D. C., Cain C. K., and Clark J. H., “Structure of Cannabidiol. VI. Isomerization of Cannabidiol to Tetrahydrocannabinol, a Physiologically Active Product. Conversion of Cannabidiol to Cannabinol,” Journal of the American Chemical Society 62, no. 9 (1940): 2402–2405, 10.1021/ja01866a040. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources