Real-world outcomes of the CROSS regimen in patients with resectable esophageal or gastro-esophageal junction adenocarcinoma: a nationwide cohort study in the Netherlands
- PMID: 39911244
- PMCID: PMC11795631
- DOI: 10.1016/j.eclinm.2024.103067
Real-world outcomes of the CROSS regimen in patients with resectable esophageal or gastro-esophageal junction adenocarcinoma: a nationwide cohort study in the Netherlands
Abstract
Background: Recent studies in patients with resectable adenocarcinoma of the esophagus or gastroesophageal junction (GEJ)-Neo-AEGIS and ESOPEC-have explored the comparison of neoadjuvant chemoradiotherapy (nCRT) with chemotherapy, with conflicting results. To contextualize the findings from these studies using nCRT as a comparator, we aimed to investigate contemporary real-world outcomes of nCRT in patients with adenocarcinoma of the esophagus or GEJ.
Methods: From the Netherlands Cancer Registry, patients were selected who were diagnosed between 1 January 2015 and 31 December 2022 with a resectable (cT1N+M0 or cT2-4aNanyM0) esophageal, GEJ or gastric cardia adenocarcinoma and started treatment with nCRT according to the CROSS regimen, that is 5 weekly cycles of carboplatin (AUC 2 mg/mL per minute) and paclitaxel (50 mg/m2) combined with concurrent radiotherapy (41.4 Gy in 23 fractions of 1.8 Gy). Pathologic complete response (pCR) according to Mandard was the primary outcome of this study and defined as complete tumor regression of the primary tumor (Mandard grade I) irrespective of residual nodal involvement.
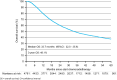
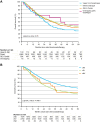
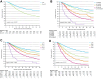
Findings: Of the 4765 included patients, 4170 (87.5%) completed the full CROSS regimen of radiotherapy and chemotherapy. A pCR was observed in 704 (20.5%) of 3439 patients who underwent surgical resection within 16 weeks after completing the CROSS regimen. In the complete study population, the median overall survival (OS) was 33.7 months (95% CI 32.0-35.6), with a 3-year OS rate of 48.1%.
Interpretation: Although survival rates in real-world settings are often lower compared to clinical trials, in our real-world cohort the 3-year OS was only 2.6% lower compared to that reported for the group that underwent nCRT in ESOPEC. These real-world results underscore the potential of the CROSS regimen in daily clinical practice.
Funding: None.
Keywords: Esophageal adenocarcinoma; Neoadjuvant chemoradiotherapy; Overall survival; Pathological complete response; Real-world data.
© 2024 The Author(s).
Conflict of interest statement
Hanneke van Laarhoven has acted as a consultant or in an advisory role for Amphera, Astellas, Beigene, Daiichy, Myeloid; and has received research funding and/or medication/material supply from AMGEN, AstraZeneca, AURISTONE, BMS, Incyte, Merck, ORCA, and Servier; and speaker roles for Astellas, AstraZeneca, BMS, Benecke, Daiichi-Sankyo, JAAP, Medtalks, Novartis, Servier, and Travel Congress Management; travel support from AstraZeneca; she is chair of the ESMO upper GI faculty. Rob Verhoeven has received research funding from BMS and Amgen and has acted as consultant for Daiichi Sankyo. Mark van Berge Henegouwen reports consulting or advisory roles for Viatris, Johnson & Johnson, BBraun, Stryker and Medtronic. Nadia Haj Mohammad has acted as a consultant or in an advisory role for BMS, Astra Zeneca, Servier, MSD, and Eli Lilly; and has received research funding and/or medication/material supply from Servier. Richard van Hillegersberg is Procotor for Intutive Surgical, member of advisory board of Medtronic, Olympus and Ethicon. Marije Slingerland has acted as a consultant or advisory role for BMS, Astra Zeneca and Lilly. Christina Muijs has no personal declarations of interest. Bianca Mostert has acted as a consultant or advisory role for BMS, Lilly, Servier, Amgen and AstraZeneca, and has received research funding and/or medication/material supply from BMS and Pfizer. Bas Wijnhoven has received research funding from BMS and has acted as consultant for Medtronic. Laurens Beerepoot has had speaker roles for Servier, BMS, Congress Care, Ipsen, Medtalks, Benecke and Travel Congress Management. Grard Nieuwenhuijzen has acted as a consultant or advisory role for Medtronic. Sarah Derks has acted as a consultant or advisory role for BMS, has received research funding and/or medication/material supply from Incyte; and speaker roles for BMS, Benecke and Servier. Peter van Rossum has no personal declarations of interest.
Figures
References
-
- van Hagen P., Hulshof M.C., van Lanschot J.J., et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. - PubMed
-
- Shapiro J., van Lanschot J.J.B., Hulshof M.C.C.M., et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. - PubMed
-
- Al-Kaabi A., van der Post R.S., van der Werf L.R., et al. Impact of pathological tumor response after CROSS neoadjuvant chemoradiotherapy followed by surgery on long-term outcome of esophageal cancer: a population-based study. Acta Oncol. 2021;60(4):497–504. - PubMed
-
- Kelly R.J., Ajani J.A., Kuzdzal J., et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–1203. - PubMed
-
- Verhoeven R., Kuijper S., Slingerland M., et al. 438P Adjuvant nivolumab after neoadjuvant chemoradiotherapy and surgery for patients with esophageal or gastroesophageal junction cancer: a nationwide real-world matched comparison of overall survival. Ann Oncol. 2024;35:S176–S177.
LinkOut - more resources
Full Text Sources

