Association of hepatitis B virus DNA levels with efficacy and safety and the impact of antiviral therapy on prognosis in liver cancer patients receiving immune checkpoint inhibitors therapy: a systematic review and meta-analysis
- PMID: 39911258
- PMCID: PMC11794511
- DOI: 10.3389/fmicb.2025.1501139
Association of hepatitis B virus DNA levels with efficacy and safety and the impact of antiviral therapy on prognosis in liver cancer patients receiving immune checkpoint inhibitors therapy: a systematic review and meta-analysis
Abstract
Background: The current evidence regarding the relationship between baseline hepatitis B virus (HBV) DNA levels and survival outcomes in liver cancer patients receiving immune checkpoint inhibitors (ICIs) remains inconsistent. Therefore, this review was intended to explore the impact of the baseline HBV-DNA level on the efficacy and safety of ICIs in patients with liver cancer.
Methods: Relevant studies were identified through a comprehensive search in PubMed, EMBASE, Cochrane Library, and Web of Science up to August 1, 2024. The outcomes were hazard ratios (HRs) for overall survival (OS) and progression-free survival (PFS), as well as odds ratios (ORs) for objective response rate (ORR), disease control rate (DCR) and HBV reactivation (HBVr). Subgroup analysis, publication bias, and sensitivity analysis were conducted with STATA 14.0.
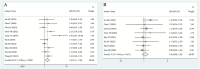
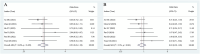
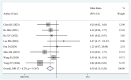
Results: This meta-analysis comprised 17 articles involving a total of 2,130 patients. The pooled results demonstrated that high HBV DNA was associated with a worse OS (HR = 1.48 95% CI 1.11-1.96). Further subgroup analysis showed that there was no difference in OS between the high HBV DNA group and low HBV DNA group when all patients received antiviral treatment. No associations between baseline HBV DNA and PFS (HR = 1.08, 95% CI 0.90-1.29), ORR (OR = 0.91, 95% CI 0.65-1.28), or DCR (OR = 0.83, 95% CI 0.58-1.20) were observed. The risk of HBVr in the high HBV DNA group was lower than that in the low HBV DNA group (OR = 0.30, 95% CI 0.15-0.58), especially among patients who received antiviral therapy (OR = 0.42, 95% CI 0.18-0.98).
Conclusion: High HBV DNA was associated with worse OS, but not with PFS, ORR, or DCR in liver cancer patients receiving ICIs. When patients were simultaneously treated with antiviral treatment, elevated HBV DNA level had no unfavorable impact on the efficacy of ICIs. Furthermore, the risk of HBVr in the high HBV-DNA group was lower than that in the low HBV DNA group. More prospective studies with larger sample sizes are essential to confirm the results.
Keywords: antiviral therapy; hepatitis B virus DNA; immune checkpoint inhibitors; liver cancer; meta-analysis.
Copyright © 2025 Cui, Li, Lv and Xiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The load of hepatitis B virus reduces the immune checkpoint inhibitors efficiency in hepatocellular carcinoma patients.Front Immunol. 2024 Nov 27;15:1480520. doi: 10.3389/fimmu.2024.1480520. eCollection 2024. Front Immunol. 2024. PMID: 39664382 Free PMC article.
-
Correlation of HBV DNA and Hepatitis B Surface Antigen Levels With Tumor Response, Liver Function and Immunological Indicators in Liver Cancer Patients With HBV Infection Undergoing PD-1 Inhibition Combinational Therapy.Front Immunol. 2022 May 25;13:892618. doi: 10.3389/fimmu.2022.892618. eCollection 2022. Front Immunol. 2022. PMID: 35711409 Free PMC article.
-
Hepatocellular Carcinoma Patients with Hepatitis B Virus Infection Exhibited Favorable Survival from Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis.Liver Cancer. 2023 Oct 30;13(4):344-354. doi: 10.1159/000534446. eCollection 2024 Aug. Liver Cancer. 2023. PMID: 39021889 Free PMC article.
-
Prognostic value of neutrophil to lymphocyte ratio in gastric cancer patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis.Front Oncol. 2023 Apr 18;13:1070019. doi: 10.3389/fonc.2023.1070019. eCollection 2023. Front Oncol. 2023. PMID: 37143942 Free PMC article.
-
Prognostic role of the platelet to lymphocyte ratio (PLR) in the clinical outcomes of patients with advanced lung cancer receiving immunotherapy: A systematic review and meta-analysis.Front Oncol. 2022 Aug 22;12:962173. doi: 10.3389/fonc.2022.962173. eCollection 2022. Front Oncol. 2022. PMID: 36059629 Free PMC article.
References
-
- An M., Wang W., Zhang J., Till B. G., Zhao L., Huang H., et al. . (2022). Association of hepatitis B virus DNA levels with overall survival for advanced hepatitis B virus-related hepatocellular carcinoma under immune checkpoint inhibitor therapy. Cancer Immunol. Immunother. 72, 385–395. doi: 10.1007/s00262-022-03254-w, PMID: - DOI - PMC - PubMed
-
- Balermpas P., Martin D., Wieland U., Rave-Fränk M., Strebhardt K., Rödel C., et al. . (2017). Human papilloma virus load and PD-1/PD-L1, CD8(+) and FOXP3 in anal cancer patients treated with chemoradiotherapy: rationale for immunotherapy. Onco Targets Ther 6:e1288331. doi: 10.1080/2162402x.2017.1288331, PMID: - DOI - PMC - PubMed
-
- Chen C., An L., Cheng Y., Luo X., Li Z., Liu X. (2020). Clinical outcomes and prognosis factors of Nivolumab plus chemotherapy or multitarget tyrosine kinase inhibitor in multi-line therapy for recurrent hepatitis B virus-related hepatocellular carcinoma: a retrospective analysis. Frontiers. Oncology 10:10. doi: 10.3389/fonc.2020.01404, PMID: - DOI - PMC - PubMed
-
- Chen Q.-J., Lin K.-Y., Lin Z.-W., Zhang B., Liu M.-Q., Zhang J.-X., et al. . (2023). Association of hepatitis B virus DNA levels with efficacy and safety outcomes in patients with hepatitis B virus-associated advanced hepatocellular carcinoma receiving tyrosine kinase inhibitor plus anti-PD-1 antibody: a multicenter propensity-matched study. Int. Immunopharmacol. 125:111098. doi: 10.1016/j.intimp.2023.111098, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources