Connecting the complexity of stereoselective synthesis to the evolution of predictive tools
- PMID: 39911341
- PMCID: PMC11791519
- DOI: 10.1039/d4sc07461k
Connecting the complexity of stereoselective synthesis to the evolution of predictive tools
Abstract
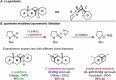
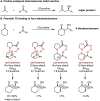
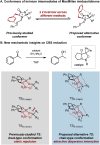
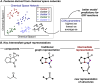
Synthetic methods have seemingly progressed to an extent where there is an apparent and increasing need for predictive models to navigate the vast chemical space. Methods for anticipating and optimizing reaction outcomes have evolved from simple qualitative pictures generated from chemical intuition to complex models constructed from quantitative methods like quantum chemistry and machine learning. These toolsets are rooted in physical organic chemistry where fundamental principles of chemical reactivity and molecular interactions guide their development and application. Here, we detail how the evolution of these methods is a successful outcome and a powerful response to the diverse synthetic challenges confronted and the innovative selectivity concepts introduced. In this review, we perform a periodization of organic chemistry focusing on strategies that have been applied to guide the synthesis of chiral organic molecules.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures














References
-
- Cram D. J. Elhafez F. A. A. J. Am. Chem. Soc. 1952;74:5828–5835. doi: 10.1021/ja01143a007. - DOI
-
- Chérest M. Felkin H. Prudent N. Tetrahedron Lett. 1968;9:2199–2204. doi: 10.1016/S0040-4039(00)89719-1. - DOI
-
- Anh N. T. Eisenstein O. Lefour J. M. Tran Huu Dau M. E. J. Am. Chem. Soc. 1973;95:6146–6147. doi: 10.1021/ja00799a068. - DOI
-
- Reetz M. T. Hüllmann M. Seitz T. Angew Chem. Int. Ed. Engl. 1987;26:477–479. doi: 10.1002/anie.198704771. - DOI
-
- Zimmerman H. E. Traxler M. D. J. Am. Chem. Soc. 1957;79:1920–1923. doi: 10.1021/ja01565a041. - DOI
Publication types
LinkOut - more resources
Full Text Sources

