Dual relay Rh-/Pd-catalysis enables β-C(sp3)-H arylation of α-substituted amines
- PMID: 39911345
- PMCID: PMC11791518
- DOI: 10.1039/d4sc06806h
Dual relay Rh-/Pd-catalysis enables β-C(sp3)-H arylation of α-substituted amines
Abstract
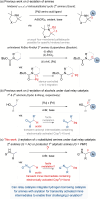
A dual relay catalytic protocol, built on reversible dehydrogenation of amines by Rh catalysis and C-H functionalisation of transient imines by Pd catalysis, is reported to enable regioselective arylation of amines at their unactivated β-C(sp3)-H bond. Notably, the new strategy is applicable to secondary anilines and N-PMP-protected primary aliphatic amines of intermediate steric demands, which is in contrast to the existing strategies that involve either free-amine-directed C-H activation for highly sterically hindered secondary aliphatic amines or steric-controlled migrative cross-coupling for unhindered N-Boc protected secondary aliphatic amines. Regioselectivity of the reaction is imposed by the electronic effects of transient imine intermediates rather than by the steric effects between specific starting materials and catalysts, thereby opening the uncharted scope of amines. In a broader sense, this study demonstrates new opportunities in dual relay catalysis involving hydrogen borrowing chemistry, previously explored in the functionalisation of alcohols, to execute otherwise challenging transformations for amines, commonly present in natural products, pharmaceuticals, biologically active molecules, and functional materials.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Transition Metal-Catalyzed Regioselective Direct C-H Amidation: Interplay between Inner- and Outer-Sphere Pathways for Nitrene Cross-Coupling Reactions.Acc Chem Res. 2022 Aug 2;55(15):2123-2137. doi: 10.1021/acs.accounts.2c00283. Epub 2022 Jul 19. Acc Chem Res. 2022. PMID: 35853135
-
A Merger of Relay Catalysis with Dynamic Kinetic Resolution Enables Enantioselective β-C(sp3)-H Arylation of Alcohols.Angew Chem Int Ed Engl. 2024 Aug 26;63(35):e202408418. doi: 10.1002/anie.202408418. Epub 2024 Jul 10. Angew Chem Int Ed Engl. 2024. PMID: 38800865
-
Iridium-Catalyzed, β-Selective C(sp3)-H Silylation of Aliphatic Amines To Form Silapyrrolidines and 1,2-Amino Alcohols.J Am Chem Soc. 2018 Dec 26;140(51):18032-18038. doi: 10.1021/jacs.8b10428. Epub 2018 Dec 13. J Am Chem Soc. 2018. PMID: 30354144 Free PMC article.
-
Application of Rh(I)/Bicyclo[2.2.1]heptadiene Catalysts to the Enantioselective Synthesis of Chiral Amines.Chem Rec. 2021 Dec;21(12):3954-3963. doi: 10.1002/tcr.202100209. Epub 2021 Oct 1. Chem Rec. 2021. PMID: 34596958 Review.
-
The azomethine ylide route to amine C-H functionalization: redox-versions of classic reactions and a pathway to new transformations.Acc Chem Res. 2015 Feb 17;48(2):317-28. doi: 10.1021/ar5003768. Epub 2015 Jan 6. Acc Chem Res. 2015. PMID: 25560649 Free PMC article. Review.
References
-
- He C. Whitehurst W. G. Gaunt M. J. Chem. 2019;5:1031–1058.
-
- Kapoor M. Singh A. Sharma K. Hua Hsu M. Adv. Synth. Catal. 2020;362:4513–4542. doi: 10.1002/adsc.202000689. - DOI
LinkOut - more resources
Full Text Sources

