Hypothalamic Estrogen Receptor α Is Essential for Female Marmoset Sexual Behavior Without Protecting From Obesity
- PMID: 39911518
- PMCID: PMC11795203
- DOI: 10.1210/jendso/bvaf012
Hypothalamic Estrogen Receptor α Is Essential for Female Marmoset Sexual Behavior Without Protecting From Obesity
Abstract
Context: Estrogen receptor α (ERα) in the ventromedial (VMN) and arcuate (ARC) nuclei of female rodent mediobasal hypothalami (MBHs) provides a crucial molecular gateway facilitating estradiol (E2) regulation of sexual behavior, reproductive neuroendocrinology, and metabolic function. In female nonhuman primates (NHPs) and women, however, its hypothalamic counterpart remains unknown.
Objective: We hypothesized that knockdown (KD) of ERα expression in the hypothalamic VMN and ARC of female marmosets would diminish sexual receptivity, while simultaneously disrupting gonadotropic and metabolic homeostasis.
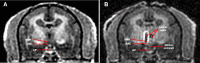
Methods: We ovariectomized (OVX) adult female marmosets of comparable age and weight, immediately replaced E2 at midcycle levels, and approximately 1 month later assigned monkeys to diet-induced obesity (DIO) within group (1) control, receiving scrambled short hairpin RNA (shRNA), or (2) ERαKD, receiving selective ERα gene silencing shRNA. Magnetic resonance imaging-guided neural surgery enabled hypothalamic infusion of viral vector shRNA and subsequent brain immunohistochemistry enabled observer-validated, NIS-elements computer software quantification of ERα knockdown.
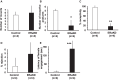
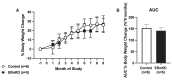
Results: ERα expression was significantly diminished in the VMN and ARC, but not the preoptic area (POA), of ERαKD females coincident with elimination of timely female sexual responses, more than 80% loss of female receptivity, modestly elevated gonadotropin levels, hyperglycemia, and diminished calorie consumption. Density and intensity of ERα-expressing cells in the VMN correlated positively with female sexual receptivity and calorie consumption, negatively with timeliness of female sexual responses, and in the ARC, correlated negatively with calorie consumption.
Conclusion: ERα activation in the female NHP MBH is critically important for female sexual behavior and modestly contributes to gonadotropic and metabolic control.
Keywords: arcuate nucleus; diet-induced obesity; female sexual dysfunction; hyperglycemia; hypergonadotropism; ventromedial nucleus.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Endocrine Society.
Figures









Similar articles
-
Role of Oestrogen α Receptors in Sociosexual Behaviour in Female Rats Housed in a Seminatural Environment.J Neuroendocrinol. 2015 Nov;27(11):803-18. doi: 10.1111/jne.12321. J Neuroendocrinol. 2015. PMID: 26314929
-
Estrogen-induced sexual incentive motivation, proceptivity and receptivity depend on a functional estrogen receptor alpha in the ventromedial nucleus of the hypothalamus but not in the amygdala.Neuroendocrinology. 2010;91(2):142-54. doi: 10.1159/000255766. Epub 2009 Nov 4. Neuroendocrinology. 2010. PMID: 19887773 Free PMC article.
-
Activation of cells containing estrogen receptor alpha or somatostatin in the medial preoptic area, arcuate nucleus, and ventromedial nucleus of intact ewes during the follicular phase, and alteration after lipopolysaccharide.Biol Reprod. 2014 Dec;91(6):141. doi: 10.1095/biolreprod.114.122408. Epub 2014 Oct 15. Biol Reprod. 2014. PMID: 25320149
-
Differential effects of site-specific knockdown of estrogen receptor α in the medial amygdala, medial pre-optic area, and ventromedial nucleus of the hypothalamus on sexual and aggressive behavior of male mice.Eur J Neurosci. 2013 Apr;37(8):1308-19. doi: 10.1111/ejn.12131. Epub 2013 Jan 24. Eur J Neurosci. 2013. PMID: 23347260
-
Norepinephrine Regulation of Ventromedial Hypothalamic Nucleus Metabolic-Sensory Neuron 5'-AMP-Activated Protein Kinase Activity: Impact of Estradiol.Int J Mol Sci. 2020 Mar 16;21(6):2013. doi: 10.3390/ijms21062013. Int J Mol Sci. 2020. PMID: 32188013 Free PMC article. Review.
References
-
- Kendrick KM, Dixson AF. The effect of the ovarian cycle on the sexual behaviour of the common marmoset (Callithrix jacchus). Physiol Behav. 1983;30(5):735‐742. - PubMed
-
- Michael RP, Richter MC, Cain JA, Zumpe D, Bonsall RW. Artificial menstrual cycles, behaviour and the role of androgens in female rhesus monkeys. Nature. 1978;275(5679):439‐440. - PubMed
-
- Wallen K, Winston LA, Gaventa S, Davis-DaSilva M, Collins DC. Periovulatory changes in female sexual behavior and patterns of ovarian steroid secretion in group-living rhesus monkeys. Horm Behav. 1984;18(4):431‐450. - PubMed
-
- Wallen K. Desire and ability: hormones and the regulation of female sexual behavior. Neurosci Biobehav Rev. 1990;14(2):233‐241. - PubMed
-
- Jones BC, Hahn AC, Fisher CI, Wang H, Kandrik M, DeBruine LM. General sexual desire, but not desire for uncommitted sexual relationships, tracks changes in women's hormonal status. Psychoneuroendocrinology. 2018;88:153‐157. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
