Calcium-mediated regulation of mitophagy: implications in neurodegenerative diseases
- PMID: 39911695
- PMCID: PMC11790495
- DOI: 10.1038/s44324-025-00049-2
Calcium-mediated regulation of mitophagy: implications in neurodegenerative diseases
Abstract
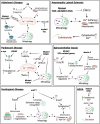
Calcium signaling plays a pivotal role in diverse cellular processes through precise spatiotemporal regulation and interaction with effector proteins across distinct subcellular compartments. Mitochondria, in particular, act as central hubs for calcium buffering, orchestrating energy production, redox balance and apoptotic signaling, among others. While controlled mitochondrial calcium uptake supports ATP synthesis and metabolic regulation, excessive accumulation can trigger oxidative stress, mitochondrial membrane permeabilization, and cell death. Emerging findings underscore the intricate interplay between calcium homeostasis and mitophagy, a selective type of autophagy for mitochondria elimination. Although the literature is still emerging, this review delves into the bidirectional relationship between calcium signaling and mitophagy pathways, providing compelling mechanistic insights. Furthermore, we discuss how disruptions in calcium homeostasis impair mitophagy, contributing to mitochondrial dysfunction and the pathogenesis of common neurodegenerative diseases.
Keywords: Metabolic disorders; Mitochondria.
© The Author(s) 2025.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures


References
-
- Giorgi, C., Marchi, S. & Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol.19, 713–730 (2018). - PubMed
-
- Berridge, M. J., Lipp, P. & Bootman, M. D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol.1, 11–21 (2000). - PubMed
-
- Rossi, A., Pizzo, P. & Filadi, R. Calcium, mitochondria and cell metabolism: a functional triangle in bioenergetics. Biochim. Biophys. Acta Mol. Cell Res.1866, 1068–1078 (2019). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
