Unveiling causal immune cell-gene associations in multiple myeloma: insights from systematic reviews and Mendelian randomization analyses
- PMID: 39911859
- PMCID: PMC11794323
- DOI: 10.3389/fmed.2025.1456732
Unveiling causal immune cell-gene associations in multiple myeloma: insights from systematic reviews and Mendelian randomization analyses
Abstract
Background: The efficacy of novel chimeric antigen receptor T-cell (CAR-T) therapy is inconsistent, likely due to an incomplete understanding of the tumor microenvironment (TME). This study utilized meta-analysis to evaluate CAR-T-cell therapy efficacy and safety and employed two-sample Mendelian randomization (MR) analysis to investigate the causal links between immune cells and Multiple Myeloma (MM).
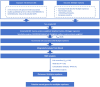
Method: Our literature review, conducted from January 1, 2019, to August 30, 2024, across Medline/PubMed, Scopus, and Web of Science, identified 2,709 articles, 34 of which met our inclusion criteria. We utilized MR analysis of GWAS data to identify immune cells causally related to multiple myeloma, followed by SMR analysis to highlight associated pathogenic genes and colocalisation analysis for validation.
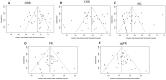
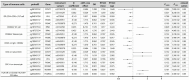
Results: The meta-analysis revealed an 82.2% overall response rate to CAR-T-cell therapy, characterized by a safe profile with a grade 3 or higher CRS of 6.3% and neurotoxicity of 0.9%. BCMA, CD38, and GPRC5D CAR-T-cell therapies had superior response rates, whereas BCMA and CD3 CAR-T-cell therapy rates lagged at 61.8%. Post-adjustment for multiple testing, the levels of seven types of immune cells (two types of Treg, two types of TNBK, two types of B cells, and one type of Myeloid cell) were found to be elevated in association with an increased risk of multiple myeloma (MM), while the levels of another eight types of immune cells (one types of Treg, three types of TNBK, one type of MT cells, and two types of Myeloid cell and one type of cDC cells) were demonstrated to be associated with a decreased risk of MM. As supported by sensitivity analysis. SMR analysis pinpointed the risk genes VDR, VHL, POMC, and FANCD2, with VHL and POMC correlating at the methylation level. VDR was not significantly correlated with MM after correction for multiple tests. NCAM1 also exhibited a significant methylation-level association with disease.
Conclusion: Our study supports the efficacy and safety of CAR-T-cell therapy in rrMM patients, with an 82.2% ORR and low rates of severe CRS (6.3%) and neurotoxicity (0.9%). This finding also suggests that BCMA/CD19 bispecific CAR-T cells have a superior ORR, pending clinical confirmation. MR analysis reveals links between immune cells, genes such as VDR and VHL, and MM, enhancing our understanding of its pathophysiology.
Keywords: Mendelian randomization; immune cells; meta-analysis; multiple myeloma; summary data-based Mendelian randomization.
Copyright © 2025 Zhang, Zhang, Lian, Kou, Zhu, Ma, Zheng and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Larrayoz M, Garcia-Barchino MJ, Celay J, Etxebeste A, Jimenez M, Perez C, et al. . Preclinical models for prediction of immunotherapy outcomes and immune evasion mechanisms in genetically heterogeneous multiple myeloma. Nat Med. (2023) 29:632–45. doi: 10.1038/s41591-022-02178-3, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

