Immunoinformatic approach to the design of a novel multi-epitope vaccine against Leishmania major fused to human IgG-Fc
- PMID: 39911897
- PMCID: PMC11792711
- DOI: 10.4103/RPS.RPS_145_24
Immunoinformatic approach to the design of a novel multi-epitope vaccine against Leishmania major fused to human IgG-Fc
Abstract
Background and purpose: Cutaneous leishmaniasis poses significant health and socioeconomic challenges, making vaccine development a top priority, especially in endemic regions. Cysteine proteases, KMP-11, and HASPB proteins are promising candidates for leishmaniasis vaccine development owing to their immunogenic properties and capacity to provoke robust immune responses, as evidenced by different investigations. This study aimed to design a recombinant chimeric protein (MEV-Fc) vaccine using multi-epitopes from these Leishmania major proteins.
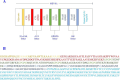
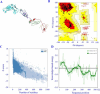
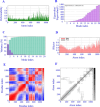
Experimental approach: The antigens were subjected to immunoinformatic prediction and screening of HTL, CTL, and B-cell epitopes. The multi-epitope protein was designed with significantly high-scoring epitopes and suitable linkers. Natural adjuvants were then added to enhance immunogenicity. Vaccine potency was innovatively improved by covalently fusing human IgG1 Fc with multi-epitope protein. To investigate how the MEV-Fc vaccine interacts with Toll-like receptors, molecular docking, multi-scale normal mode analysis simulation, and computational immune simulation were employed to study humoral and cellular immune responses.
Findings/results: The results demonstrated the vaccine's antigenicity, stability, and nontoxicity. The structural validation confirmed the accuracy of the 3D models, indicating robust interactions with TLR2 and TLR4, with binding free energies of -1269.9 and -1128.7 (kcal/mol), respectively. Immune simulation results showed significant increases in IgM and IgG antibody levels following three vaccinations, along with enhanced activation of B cells, helper T cells, and cytotoxic T lymphocytes.
Conclusion and implications: These findings provide novel insights for developing effective candidates for cutaneous leishmaniasis vaccines. However, laboratory experiments are necessary to evaluate its protective effects.
Keywords: Adjuvant; IgG-Fc; Immunodominant epitopes; Leishmania major; Subunit vaccine.
Copyright: © 2024 Research in Pharmaceutical Sciences.
Conflict of interest statement
All authors declared no conflict of interest in this study.
Figures

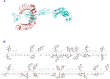

Similar articles
-
Designing a broad-spectrum multi-epitope subunit vaccine against leptospirosis using immunoinformatics and structural approaches.Front Immunol. 2025 Jan 28;15:1503853. doi: 10.3389/fimmu.2024.1503853. eCollection 2024. Front Immunol. 2025. PMID: 39936152 Free PMC article.
-
Design of a multi-epitope vaccine against goatpox virus using an immunoinformatics approach.Front Cell Infect Microbiol. 2024 Feb 29;13:1309096. doi: 10.3389/fcimb.2023.1309096. eCollection 2023. Front Cell Infect Microbiol. 2024. PMID: 38487680 Free PMC article.
-
Designing a multi-epitope subunit vaccine against Toxoplasma gondii through reverse vaccinology approach.Mol Biochem Parasitol. 2024 Dec;260:111655. doi: 10.1016/j.molbiopara.2024.111655. Epub 2024 Nov 7. Mol Biochem Parasitol. 2024. PMID: 39521441
-
A multi-epitope subunit vaccine based on CU/ZN-SOD, OMP31 and BP26 against Brucella melitensis infection in BALB/C mice.Int Immunopharmacol. 2024 Jan 25;127:111351. doi: 10.1016/j.intimp.2023.111351. Epub 2023 Dec 19. Int Immunopharmacol. 2024. PMID: 38113688
-
Design of multi-epitope vaccine against porcine rotavirus using computational biology and molecular dynamics simulation approaches.Virol J. 2024 Jul 22;21(1):160. doi: 10.1186/s12985-024-02440-9. Virol J. 2024. PMID: 39039549 Free PMC article.
References
LinkOut - more resources
Full Text Sources
