Neoadjuvant atezolizumab plus bevacizumab prior liver transplantation for hepatocellular carcinoma
- PMID: 39911942
- PMCID: PMC11794155
- DOI: 10.1016/j.jhepr.2024.101246
Neoadjuvant atezolizumab plus bevacizumab prior liver transplantation for hepatocellular carcinoma
Abstract
Background & aims: The combination of atezolizumab and bevacizumab offers a novel approach to immunomodulation, showing efficacy as a primary treatment in advanced hepatocellular carcinoma (HCC). Concerns about graft safety and rejection have limited its exploration in the neoadjuvant setting of liver transplantation (LT). In this study, we investigate the clinical efficacy and the safety profile of pre-transplant administration of atezolizumab and bevacizumab for HCC.
Methods: Herein, we performed a prospective assessment of 17 patients with HCC treated with neoadjuvant preoperative atezolizumab and bevacizumab prior to LT for HCC, obtained from December 2020 and December 2023 at seven Western transplant centers.
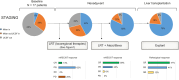
Results: Among the 17 patients with HCC included in the study, 16 (94.1%) had a tumor burden outside of Milan criteria. Neoadjuvant locoregional therapies along with the administration of atezolizumab plus bevacizumab (median: 5 months; discontinued at least 4 weeks prior to LT) led to an objective response rate of 94% (complete response: 59%), downstaging to within Milan criteria (82%) and a pathological response at explant examination of 88%. Grade 3-4 treatment-related adverse events accounted for 17.6% of cases and were manageable. During the 25-month median follow-up period, two cases of mild (rejection activity index ≤4), biopsy-proven rejection were reported but no instances of severe allograft rejection or graft loss were reported. The 1-year and 3-year post-LT survival rates were 94.2% and 88.2%, respectively.
Conclusions: This study highlights the favorable oncological and survival outcomes associated with atezolizumab and bevacizumab treatment in the pre-LT setting. This immune-based combination was safe in terms of treatment-related adverse events, and absence of severe post-transplant rejection or graft loss. These preliminary results could pave the way for expanding transplant eligibility criteria in patients at more advanced HCC stages.
Impact and implications: Studies on the combination of atezolizumab and bevacizumab in the neoadjuvant setting prior to liver transplantation for hepatocellular carcinoma have been limited, despite its potential to enhance anti-tumor responses and downstaging, owing to concerns about its safety profile. Among 17 patients who underwent successful liver transplantation following neoadjuvant atezolizumab/bevacizumab, 82% achieved downstaging to within Milan criteria, 94% radiological objective response and 88% pathology response, without drop-outs due to treatment-related adverse events or graft loss. The neoadjuvant combination of atezolizumab plus bevacizumab prior to liver transplantation for hepatocellular carcinoma shows an encouraging safety profile and stands out as a promising pre-transplant optimization treatment, leading to improved oncological outcomes.
Keywords: Downstaging; Immune Checkpoint Inhibitors; atezolizumab; bevacizumab; hepatocellular carcinoma; immunotherapy; liver transplantation.
© 2024 The Authors.
Conflict of interest statement
J.M.L received research support from Eisai Inc, Bayer HealthCare Pharmaceuticals, and Sagimet, and consulting fees from Eisai Inc, Merck, Genentech, Roche, AstraZeneca, Bayer HealthCare Pharmaceuticals, Moderna, Bristol-Myers Squibb, Exelixis and Glycotest. M.I. received consulting fees, travel grants or lectures honoraria from EISAI, IPSEN, MSD, Gilead, Astra-Zeneca, Roche, Roche Diagnostics. P.T received lecture honoraria from Astra-Zeneca, Bayer, Boston Scientific. All others no COI. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- Mehta N., Frenette C., Tabrizian P., et al. Downstaging outcomes for hepatocellular carcinoma: results from the multicenter evaluation of reduction in tumor size before liver transplantation (MERITS-LT) consortium. Gastroenterology. 2021;161(5):1502–1512. doi: 10.1053/j.gastro.2021.07.033. - DOI - PMC - PubMed
-
- Mazzaferro V., Citterio D., Bhoori S., et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial [published correction appears in Lancet Oncol. 2020 Aug;21(8):e373] Lancet Oncol. 2020;21(7):947–956. doi: 10.1016/S1470-2045(20)30224-2. - DOI - PubMed
-
- Duvoux C., Roudot-Thoraval F., Decaens T., et al. Liver Transplantation French Study Group Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012 Oct;143(4):986–994. doi: 10.1053/j.gastro.2012.05.052. e3; quiz e14-5. Epub 2012 Jun 29. PMID: 22750200. - DOI - PubMed

