Updated overall survival in patients with prior checkpoint inhibitor therapy in the phase III TIVO-3 study
- PMID: 39912344
- PMCID: PMC11799859
- DOI: 10.1093/oncolo/oyae369
Updated overall survival in patients with prior checkpoint inhibitor therapy in the phase III TIVO-3 study
Abstract
Background: The phase III TIVO-3 study demonstrated improvement in progression-free survival (PFS) with tivozanib compared with sorafenib in patients with 2-3 prior systemic regimens for metastatic renal cell carcinoma (mRCC).
Methods: The TIVO-3 trial enrolled patients with measurable mRCC who had received 2 or more prior systemic therapies, including a vascular endothelial growth factor tyrosine kinase inhibitor (VEGF-TKI). Patients were stratified by International Metastatic RCC Database Consortium risk score and type of prior treatment and were randomized 1:1 to receive tivozanib or sorafenib. Efficacy was assessed using Response Evaluation Criteria in Solid Tumors version 1.1 criteria, with PFS as the primary endpoint. Safety was evaluated using Common Terminology Criteria for Adverse Events version v4.03, and statistical analyses included Cox regression for overall survival (OS) and descriptive statistics for duration of response (DOR). The current post-hoc long-term follow-up analysis consists of an assessment of OS in the previously stratified subpopulation of patients with prior CPI exposure.
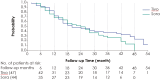
Results: Between May 2016, and August 2017, 350 patients were randomized, of which 26% had prior CPI exposure, with final analysis data cut off on June 21, 2021. In patients previously treated with CPIs (n = 91), the median PFS of tivozanib was 7.3 months versus 5.1 months with sorafenib and hazard ratio (HR) of 0.55 (95% CI, 0.32-0.94). The OS HR in the CPI-treated subset was 0.69 (95% CI, 0.43-1.11, P =.0992) favoring tivozanib, although with a median OS of 18.1 and 20.9 months, for tivozanib and sorafenib, respectively. Tivozanib demonstrated a longer median DOR of 20.3 versus 5.7 months for sorafenib in the subset previously treated with CPIs. The safety profile favored tivozanib, with lower rates of VEGF-TKI class-related grade ≥3 adverse events compared with sorafenib. However, in the subset of patients previously treated with CPIs, the incidence of grade ≥3 adverse events was higher, at 58% for tivozanib and 67% for sorafenib, compared with the ITT population, at 46% and 55%, respectively.
Conclusions: In this long-term post-hoc update of the TIVO-3 trial, we show that in CPI-resistant mRCC, the PFS benefit of tivozanib over sorafenib is accompanied with improved OS data, although not statistically significant, and durable responses.
Keywords: immunotherapy; metastatic renal cell carcinoma; sorafenib; tivozanib; vascular endothelial growth factor inhibitor.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
Figures
References
-
- Motzer RJ, Tannir NM, McDermott DF, et al.; CheckMate 214 Investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277-1290. https://doi.org/10.1056/NEJMoa1712126 - DOI - PMC - PubMed
-
- Powles T, Plimack ER, Soulières D, et al.Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21:1563-1573. https://doi.org/10.1016/S1470-2045(20)30436-8 - DOI - PubMed
-
- Choueiri TK, Eto M, Motzer R, et al.Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol. 2023;24:228-238. https://doi.org/10.1016/S1470-2045(23)00049-9 - DOI - PubMed
-
- Choueiri TK, Powles T, Burotto M, et al.; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384:829-841. https://doi.org/10.1056/NEJMoa2026982 - DOI - PMC - PubMed
-
- Porta C, Tortora G, Linassier C, et al.Maximising the duration of disease control in metastatic renal cell carcinoma with targeted agents: an expert agreement. Med Oncol. 2012;29:1896-1907. https://doi.org/10.1007/s12032-011-0016-8 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical